TOPICS
Atoms, Elements and Compounds
- ATOMS are incredibly SMALL, and make up EVERYTHING in the universe.
- They are the SMALLEST part of an ELEMENT that can exist.
- They are composed of THREE even smaller particles known as SUB-ATOMIC PARTICLES: PROTONS, NEUTRONS, and ELECTRONS.
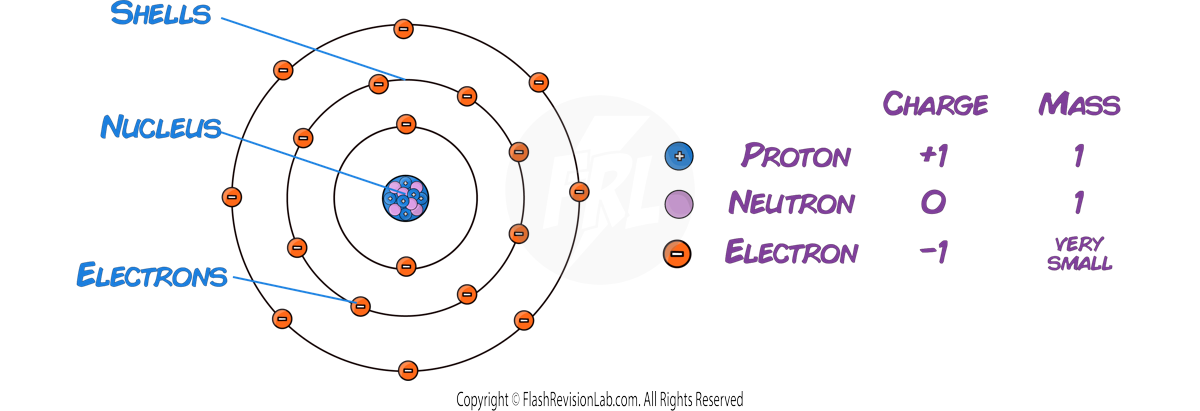
- At the center of an atom lies the NUCLEUS, containing PROTONS and NEUTRONS.
- PROTONS have a positive charge (+1) and a relative mass of 1.
- NEUTRONS have no charge (0) and a relative mass of 1, similar to protons.
- ELECTRONS orbit around the nucleus in electron shells.
- They are negatively charged (−1) and are VERY SMALL, with virtually no mass when compared to protons and neutrons.
- The NUCLEUS is tiny compared to the whole atom, about 1/10,000th of the atom's total size, but it holds nearly ALL the atom's mass.
Radius of an ATOM: 0.1nm (1 x 10-10 m)
Radius of a NUCLEUS: 1 x 10-14 m
- Atoms are NEUTRAL and have NO OVERALL CHARGE.
- This is because they have the SAME NUMBER OF PROTONS AND ELECTRONS, which CANCELS OUT THEIR CHARGES.
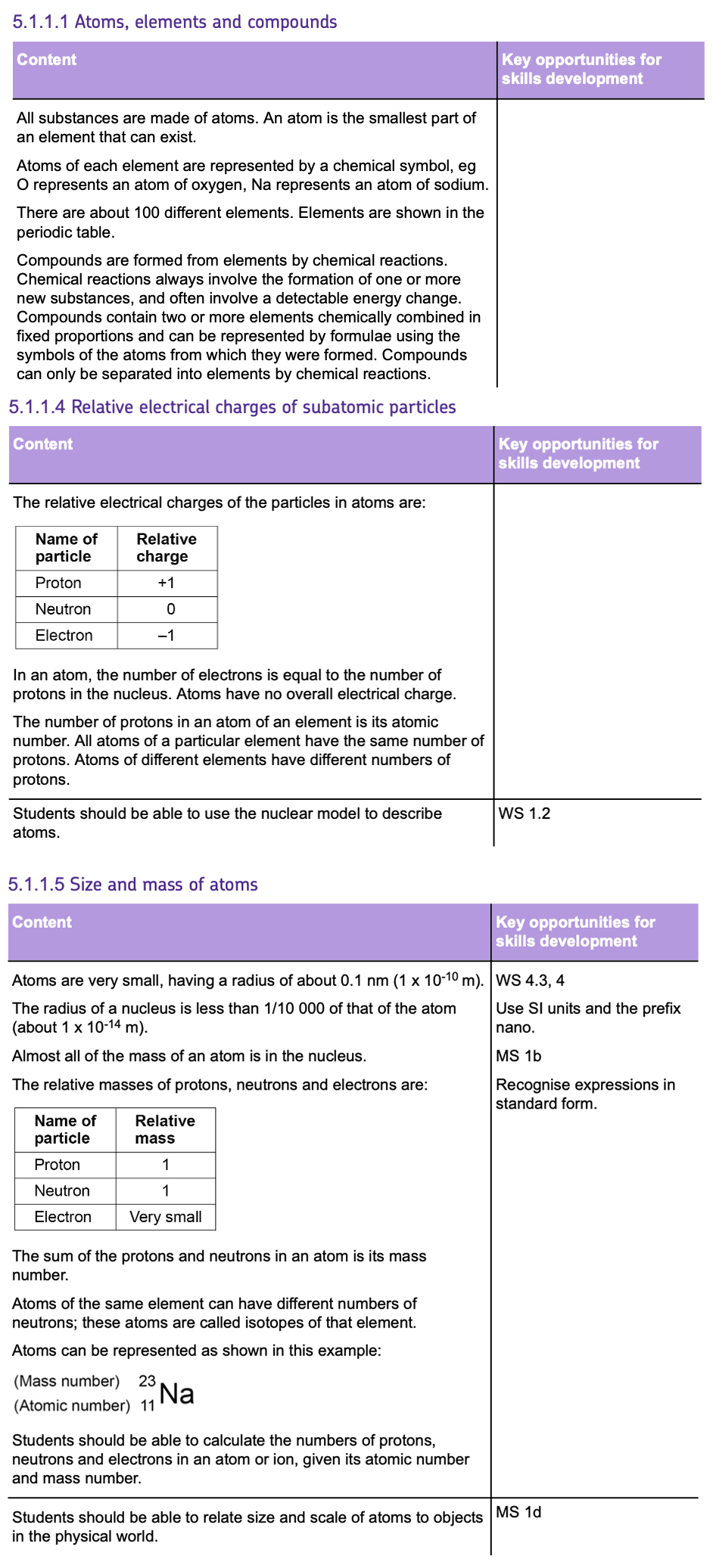
Chemical Symbols
- Atoms of each element are represented by a CHEMICAL SYMBOL which can be found in the PERIODIC TABLE. E.g. Carbon has the symbol C.
- They can be represented as below:
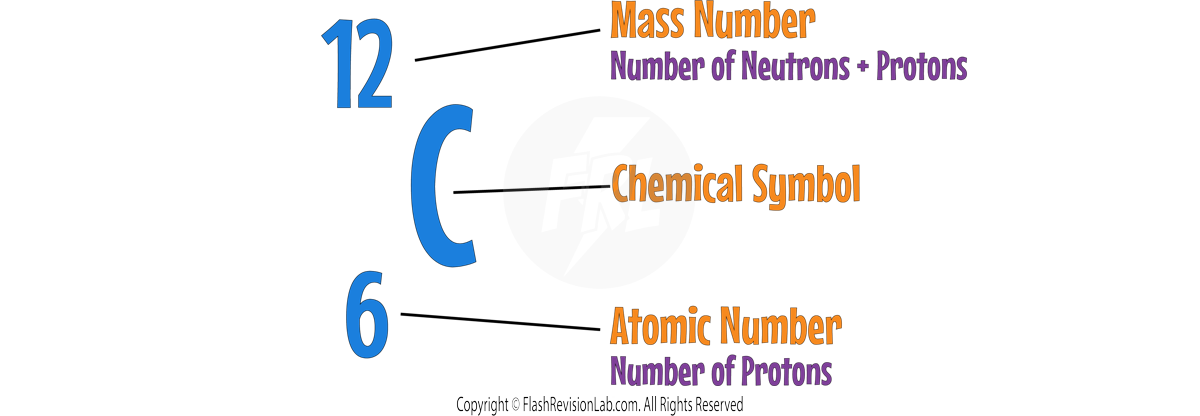
- The ATOMIC NUMBER indicates how many protons are in an atom's nucleus.
- The MASS NUMBER is the total count of both protons and neutrons.
- To find the number of NEUTRONS, SUBTRACT the atomic number from the mass number.
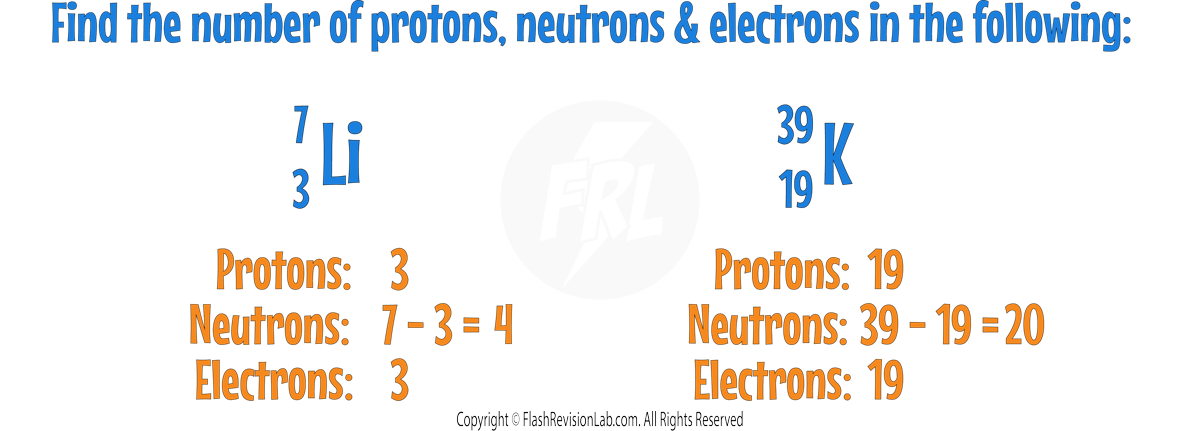
Elements:
- An ELEMENT is a substance made up of ONE TYPE OF ATOM.
- There are over 100 different discovered ELEMENTS which can all be found in the PERIODIC TABLE.
- All the atoms in an element contain the SAME NUMBER of PROTONS in their nucleus.
- When atoms have different numbers of PROTONS, they are classified as DIFFERENT elements.
- This means the ATOMIC NUMBER determines the ELEMENT of an atom. For example, Carbon atoms ALWAYS have 12 protons (has an atomic number of 12).
Isotopes
- All atoms of a particular ELEMENT have the same ATOMIC NUMBER but may have different numbers of NEUTRONS.
- ISOTOPES are different forms of the same element, having the SAME NUMBER OF PROTONS (same atomic number) but DIFFERENT NUMBERS OF NEUTRONS (different mass numbers).
- A common example is Carbon-12, Carbon-13 and Carbon-14 which all have six protons but differ in the number of neutrons:

Compounds
- COMPOUNDS are substances that form when two or more elements react and their atoms combine.
- These elements join in FIXED PROPORTIONS and are held together by CHEMICAL BONDS.
- In compounds, only the ELECTRONS are involved in bond formation while the nuclei remain unchanged.
- The PROPERTIES of a compound are usually quite different from the properties of the original elements.
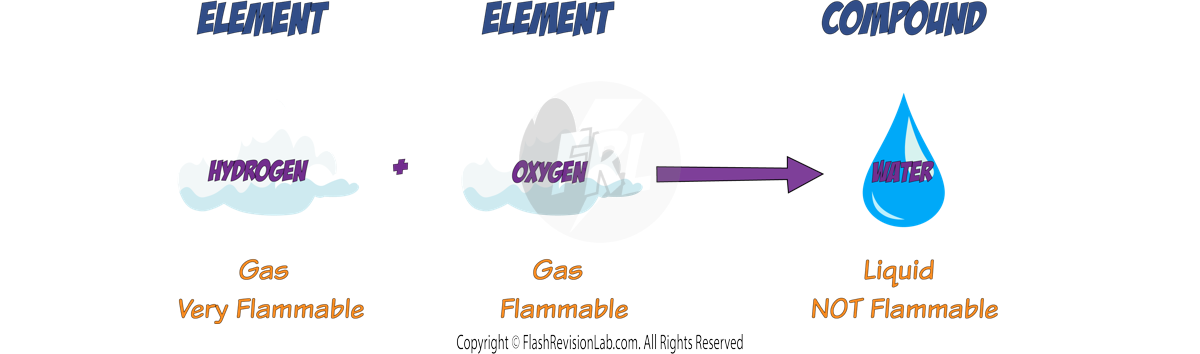
- For example, HYDROGEN is a colourless, odourless gas that is highly flammable, while OXYGEN is a colourless gas that can burn (combustion). When these two elements react, they form WATER (H₂O) which is a liquid at room temperature and does NOT BURN. The properties of water are entirely different from those of its constituent gases, hydrogen and oxygen.
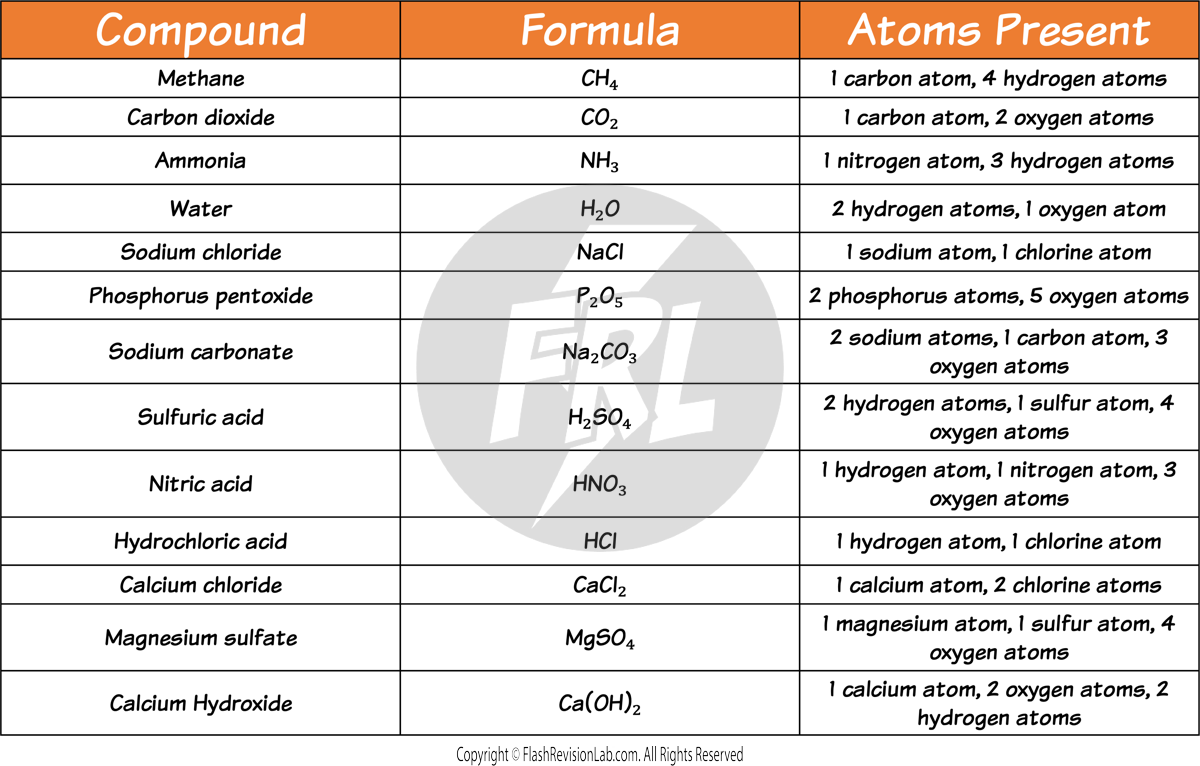
Compound Formulas
- FORMULAS represent compounds and show the elements and the number of atoms of each element in a compound.
- The formula for water is H₂O, indicating 2 hydrogen atoms and one oxygen atom.
- Some familiar compounds and their formulas include:
Relative Atomic Mass
Each element that exists has its own RELATIVE ATOMIC MASS and they are found in the periodic table as the bigger number.
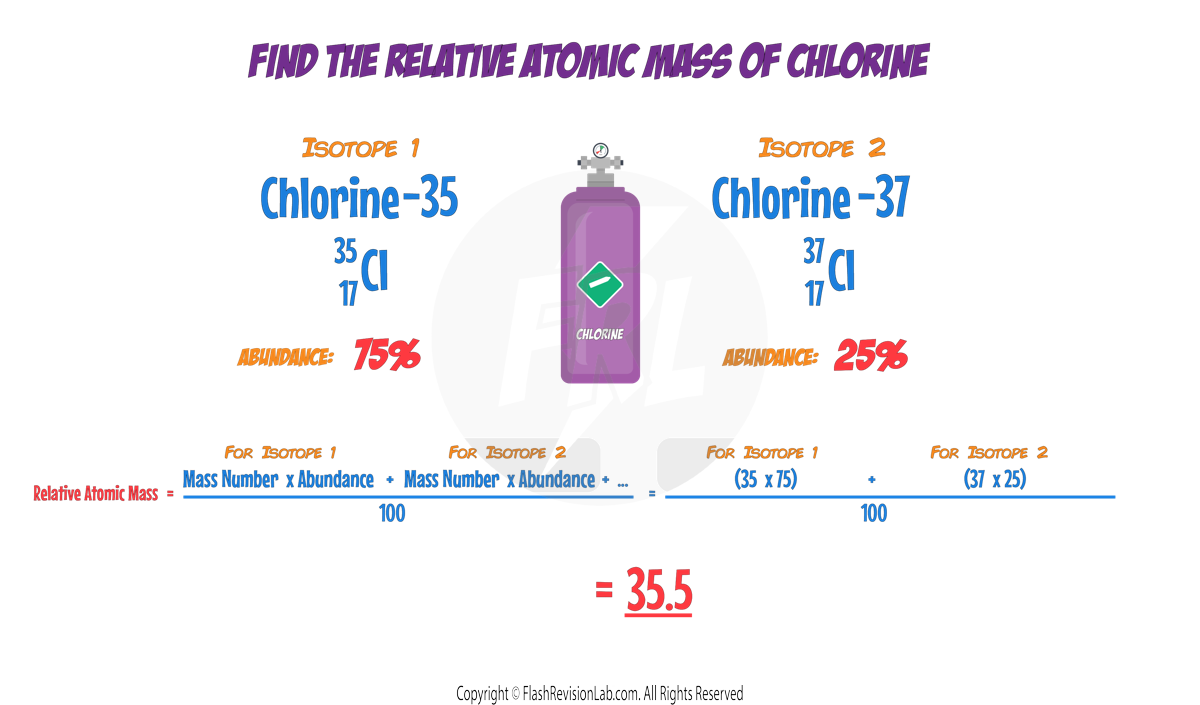
- The RELATIVE ATOMIC MASS (Ar) of an element is calculated as an average mass of all its ISOTOPES, taking into account their ABUNDANCE.
- The ABUNDANCE of an element is HOW COMMON a particular isotope is.
Calculating Relative Atomic Mass
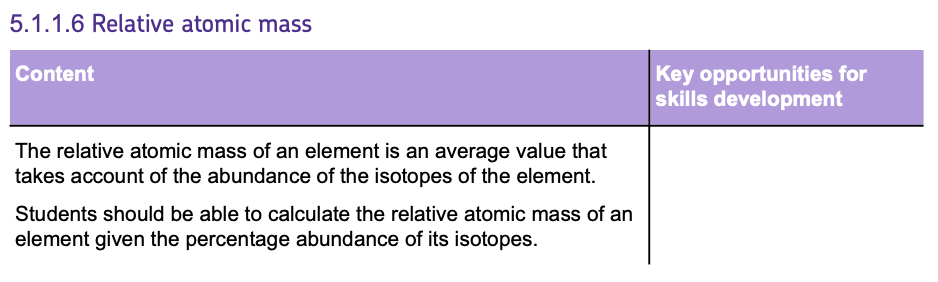
- To calculate the relative atomic mass, use the formula:

As an example let's calculate the Relative Atomic Mass of Chlorine given that there are TWO isotopes present. Chlorine-35 and Chlorine-37 which both have abundances of 75% and 25% respectively.
Chemical Equations
CHEMICAL EQUATIONS are used to show what happens during a chemical reaction. The LEFT hand side represents the REACTANTS and the RIGHT hand side represents the PRODUCTS.
- A WORD EQUATION expresses the reactants and products of a reaction in WORDS.
- A SYMBOL EQUATION is more CONCISE, using chemical symbols and FORMULAS to represent the reactants and products. For example, methane burning in oxygen can be written as:

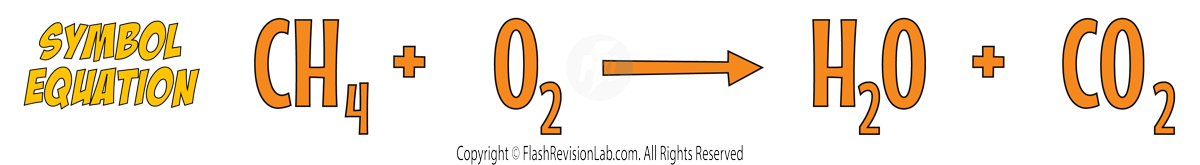
However the symbol equation is NOT correct as it needs to be BALANCED.
Balancing Chemical Equations
- Symbol equations must be BALANCED, meaning they have the same number of each type of atom on both sides of the equation.
- To balance an equation, COEFFICIENTS (numbers in front of the formulas) are adjusted so that the number of ATOMS for each element is equal on both sides.
- The CONSERVATION OF MASS tells us that no atoms are LOST or GAINED in a chemical reaction, they are just REARRANGED.
- This means the equation needs to be BALANCED.
- To balance an equation you ADJUST the COEFFICIENTS until there are the SAME number of ATOMS of the SAME ELEMENT on each side.
Steps to Balance Equations
- Identify the number of atoms of EACH ELEMENT on either side of the equation.
- ADJUST the coefficients to balance that element, which may cause another element to become unbalanced.
- Repeat the process, adjusting coefficients as necessary until the equation is BALANCED.
Consider the reaction with methane and oxygen:
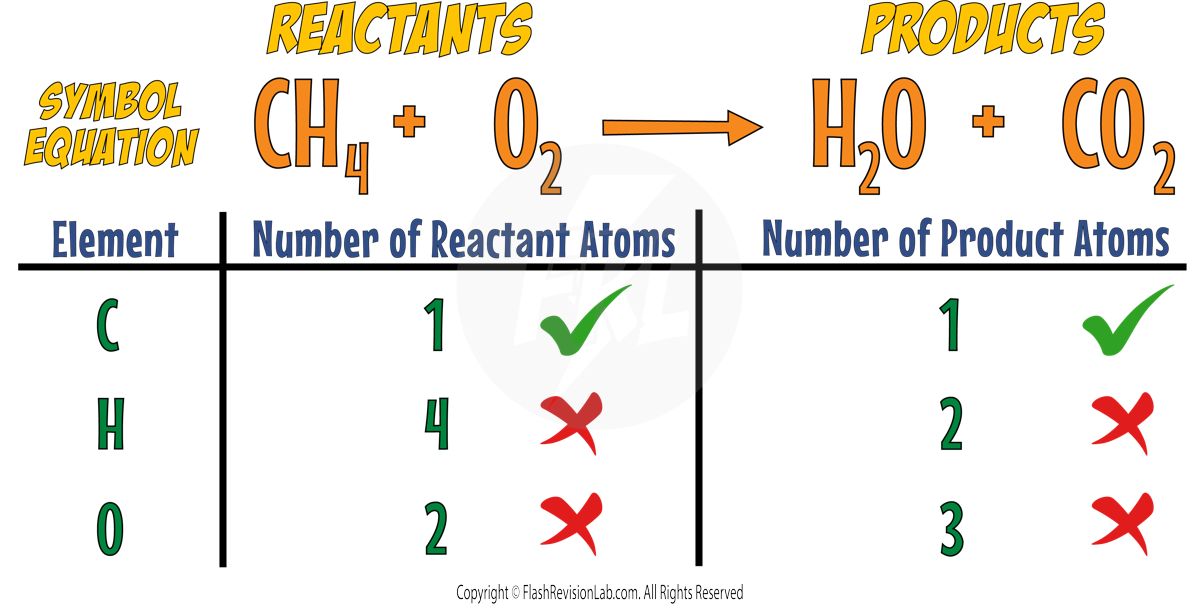
The Carbons are both balanced but the Hydrogens and the Oxygens are not.
You can put a 2 in front of WATER to make the reaction have 4 HYDROGENS on BOTH SIDES.
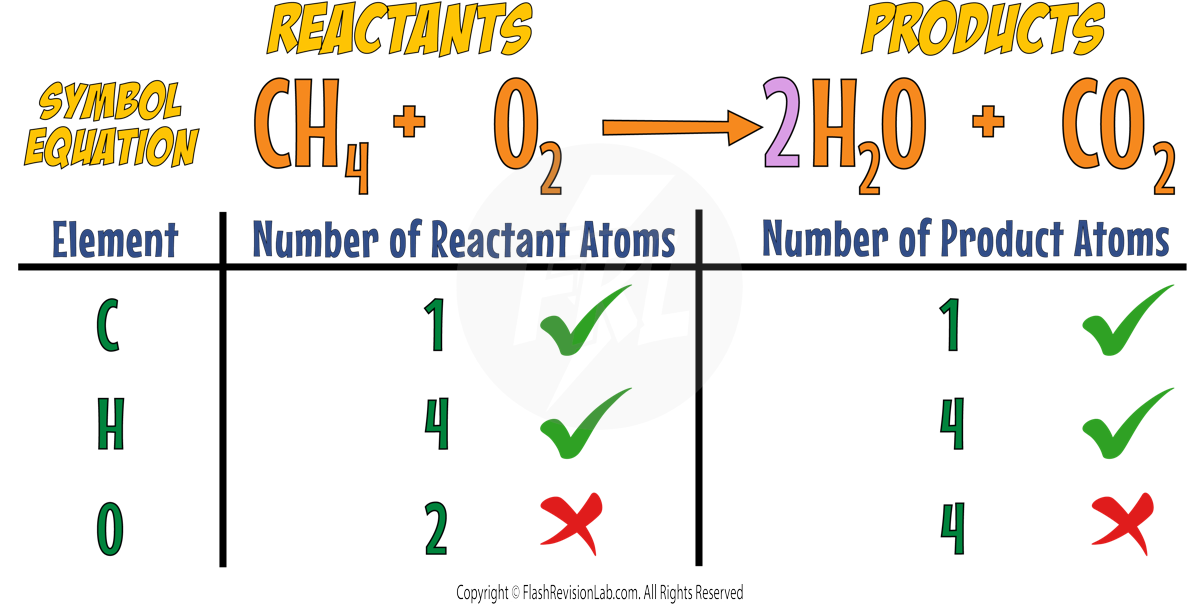
Now the Carbons and Hydrogens are both BALANCED but the Oxygens are NOT.
You can put a 2 in front of the OXYGEN to balance it.
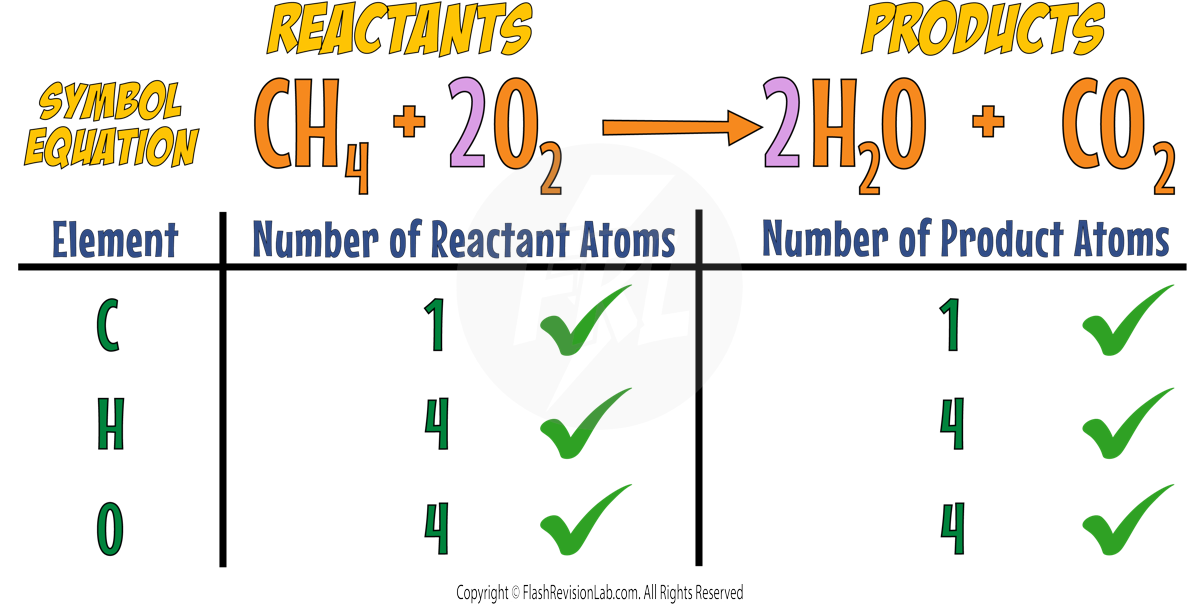
Now the equation is BALANCED.
Separating Mixtures
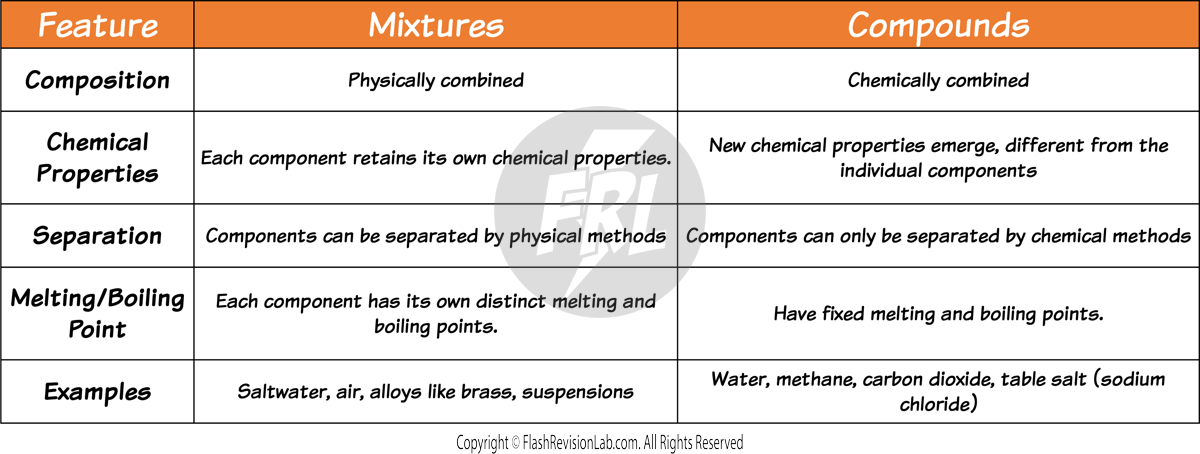
MIXTURES
- Mixtures AND Compounds are both combinations of TWO or MORE SUBSTANCES.
- COMPOUNDS are combinations of two or more substances that are CHEMICALLY COMBINED.
- MIXTURES are combinations of two or more substances that are NOT CHEMICALLY COMBINED.
- The substances in a mixture can be both ELEMENTS or COMPOUNDS, and they RETAIN their individual PROPERTIES.
- Compounds can only be separated using CHEMICAL REACTIONS.
- Mixtures can be separate using PHYSICAL METHODS.
SEPARATING MIXTURES
The components of mixtures can often be separated more easily than compounds because they are NOT CHEMICALLY BONDED.
Examples of Physical Separation Techniques include:
- FILTRATION
- EVAPORATION
- CRYSTALLISATION
- SIMPLE DISTILLATION
- FRACTIONAL DISTILLATION
- CHROMATOGRAPHY
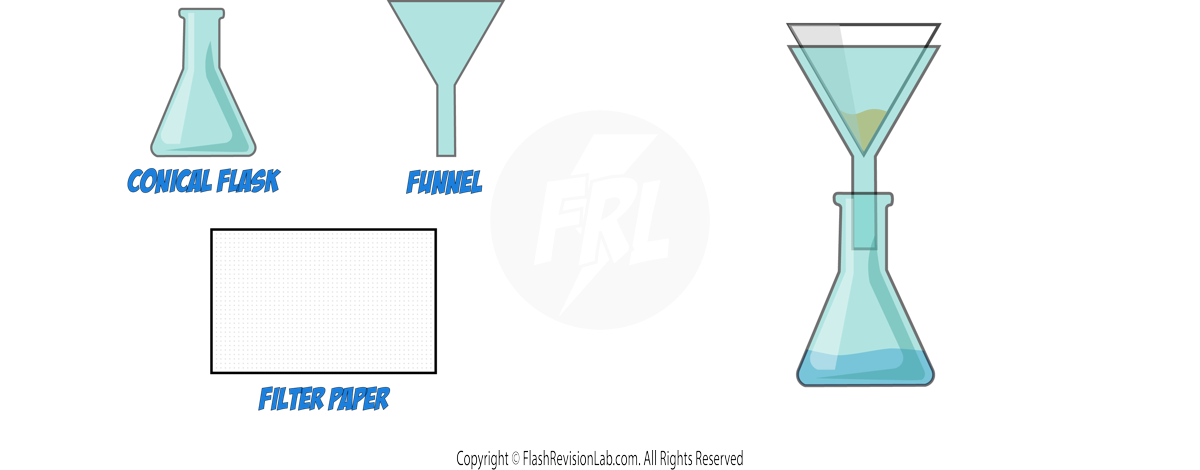
1. FILTRATION:
FILTRATION is a method used to separate an INSOLUBLE SOLID from a liquid. This can be particularly useful for removing solid impurities from a reaction mixture or product.
- The process involves pouring the mixture through a FILTER PAPER, where the solid is trapped and the liquid passes through.
2. EVAPORATION & CRYSTALLISATION:
- SOLUBLE SOLIDS, like salt, can be dissolved in a SOLVENT and then recovered by EVAPORATION or CRYSTALLISATION.
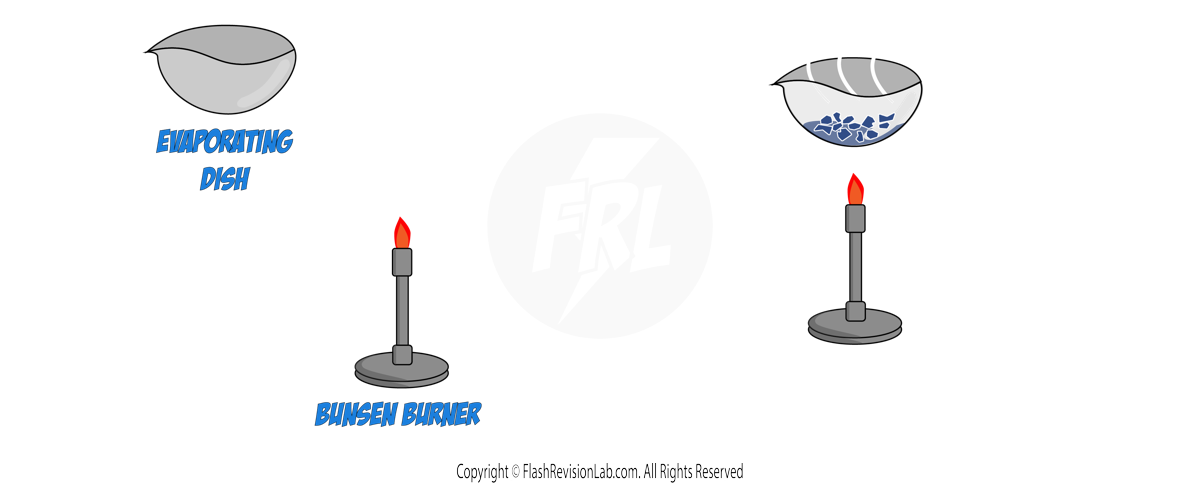
a) Evaporation:
- Pour the solution into an EVAPORATING DISH and gently heat it.
- As the solvent evaporates, the solution becomes more concentrated, and eventually, dry crystals will form.
- This method is best used when the substance DOES NOT DECOMPOSE upon heating.
b) Crystallisation:
- Similar to evaporation, start with a solution in an evaporating dish.
- HEAT gently until crystals start to form, indicating that the solution has become saturated.
- Allow the solution to COOL, which causes the formation of more crystals.
- Filter out the crystals and leave them to DRY in a warm place.
3. DISTILLATION:
a) Simple Distillation:
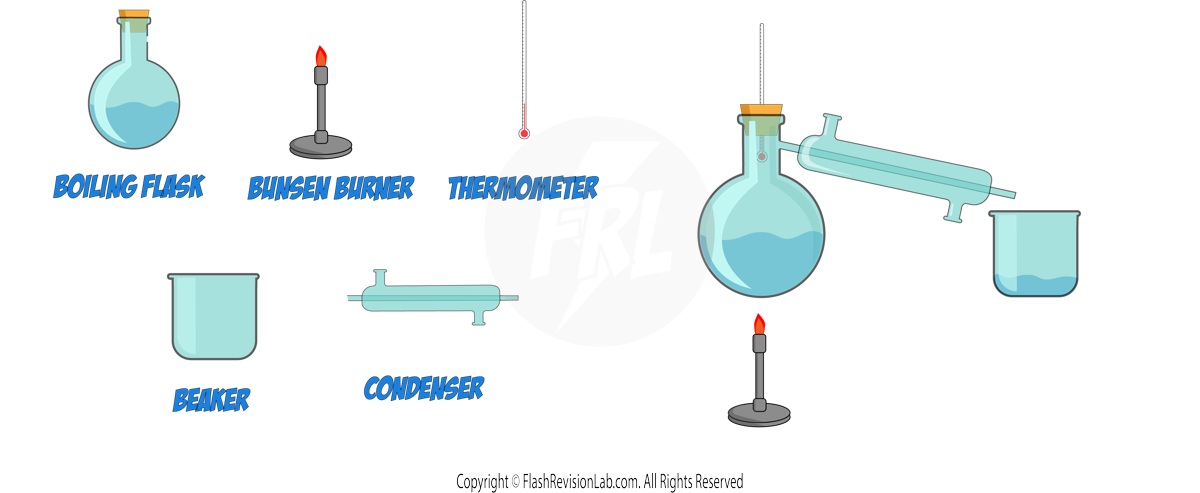
- SIMPLE DISTILLATION is a process to separate a LIQUID from a solution, often used when the liquids have different BOILING POINTS.
- During distillation, the solution is heated to the temperature of the LOWEST BOILING POINT. This causes the component with the lower boiling point to EVAPORATE.
- The vapour is then COOLED in a CONDENSER and collected as a liquid, leaving the component with the higher boiling point behind.
- This method is ideal for purifying water from seawater or separating a liquid product from a reaction mixture.
b) Fractional Distillation:

- FRACTIONAL DISTILLATION is used when you have a mixture of MORE THAN TWO liquids that have different boiling points.
- It involves using a FRACTIONATING COLUMN with glass rods in it, which allows substances with different boiling points to separate effectively. It creates a TEMPERATURE GRADIENT where the temperature is HIGH at the BOTTOM and LOW at the TOP.
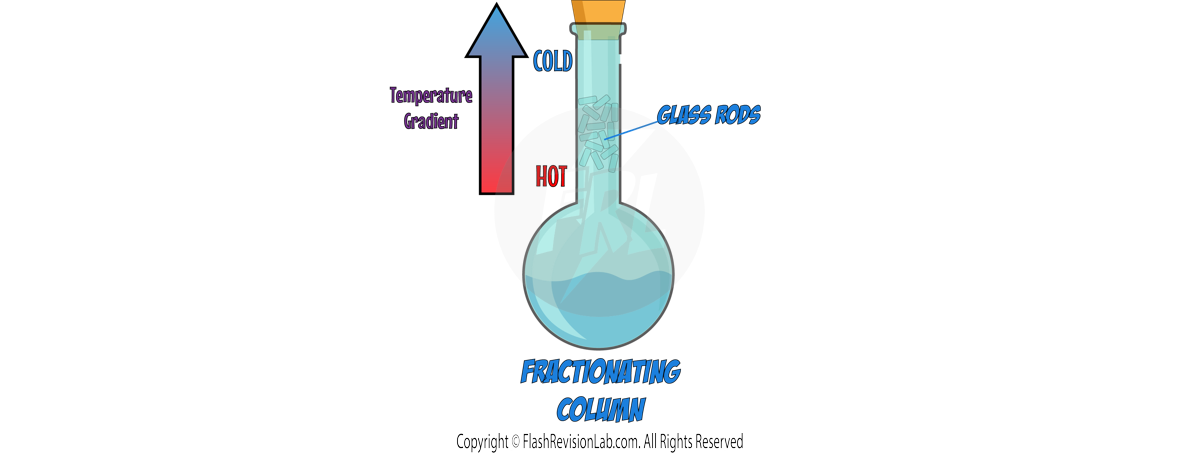
- As the vapour of the mixture rises, the temperature cools down which causes the components with HIGHER BOILING POINTS to CONDENSE and fall back into the flask.
- The component with the LOWEST BOILING POINT will rise and condense into liquid and get collected in the beaker.
- The process is repeated, raising the temperature gradually to collect other liquids at different boiling points.
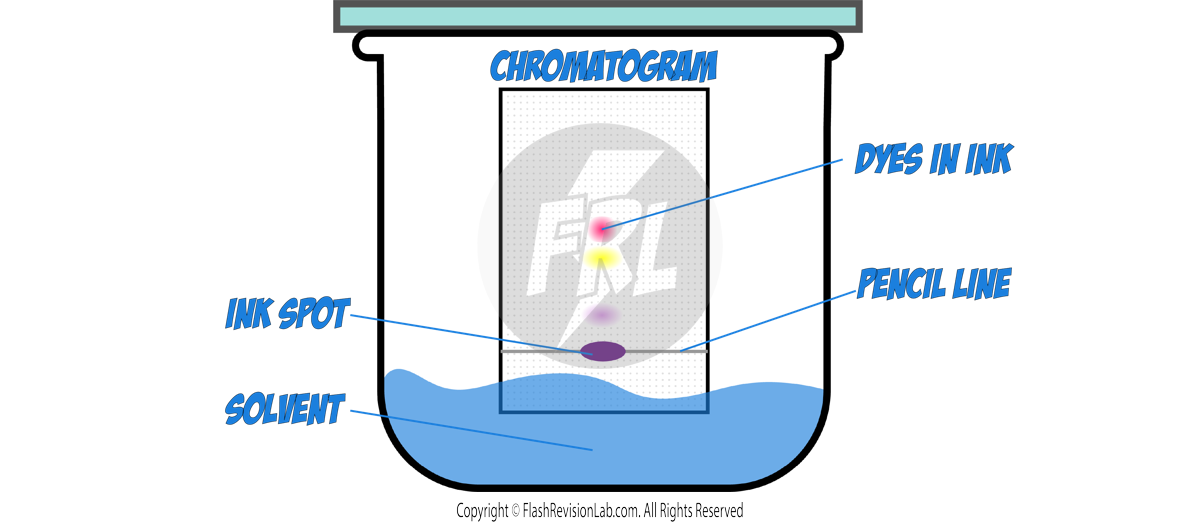
4. CHROMATOGRAPHY:
- CHROMATOGRAPHY is a method used to separate and analyse the components of a mixture.
- In paper chromatography, substances move at different rates, allowing them to be separated based on their solubility.
- It can be used to separate out the DYES in an INK.
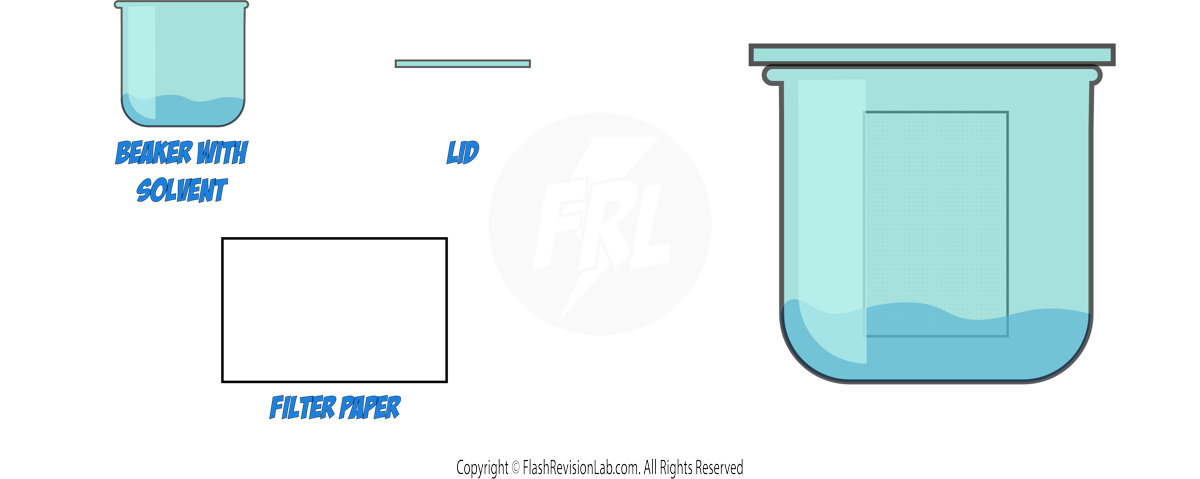
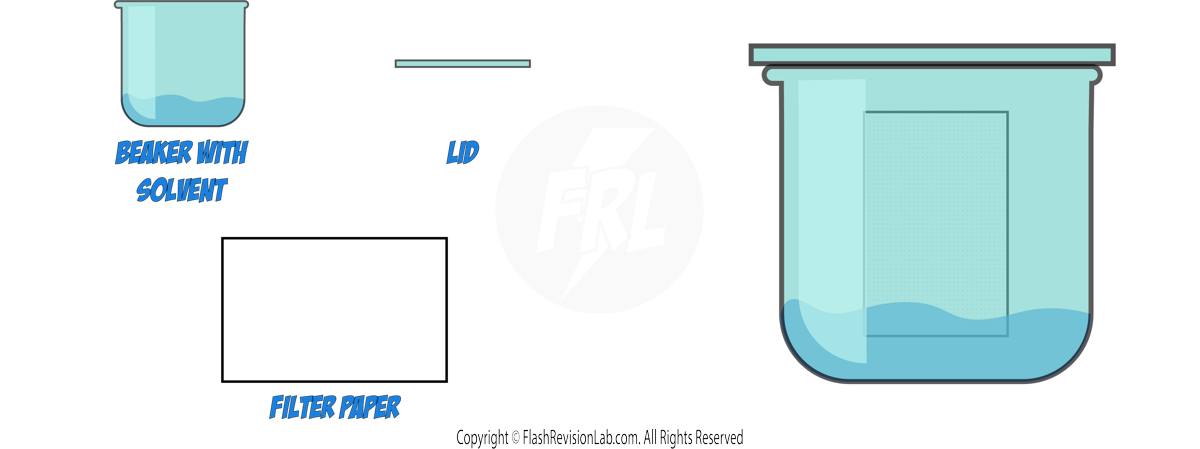
How to Perform Paper Chromatography:
- Draw a line near the bottom of the filter paper with a pencil, as pencil marks are INSOLUBLE.
- Place a spot of the substance to be separated on the line.
- Use a suitable SOLVENT, such as water or ethanol, depending on the substances.
- Ensure the spot does not touch the solvent when you place the filter paper in the beaker.
- The solvent will move up the paper, taking the substances with it.
- Different substances in the mixture will travel at different speeds, resulting in separate spots.
- Once the solvent has nearly reached the top, remove the paper and let it dry to see the CHROMATOGRAM.
Development of the Model of the Atom
As technology improved and we discovered more information about particles, the model of what we think an atom looks like has changed significantly over time.
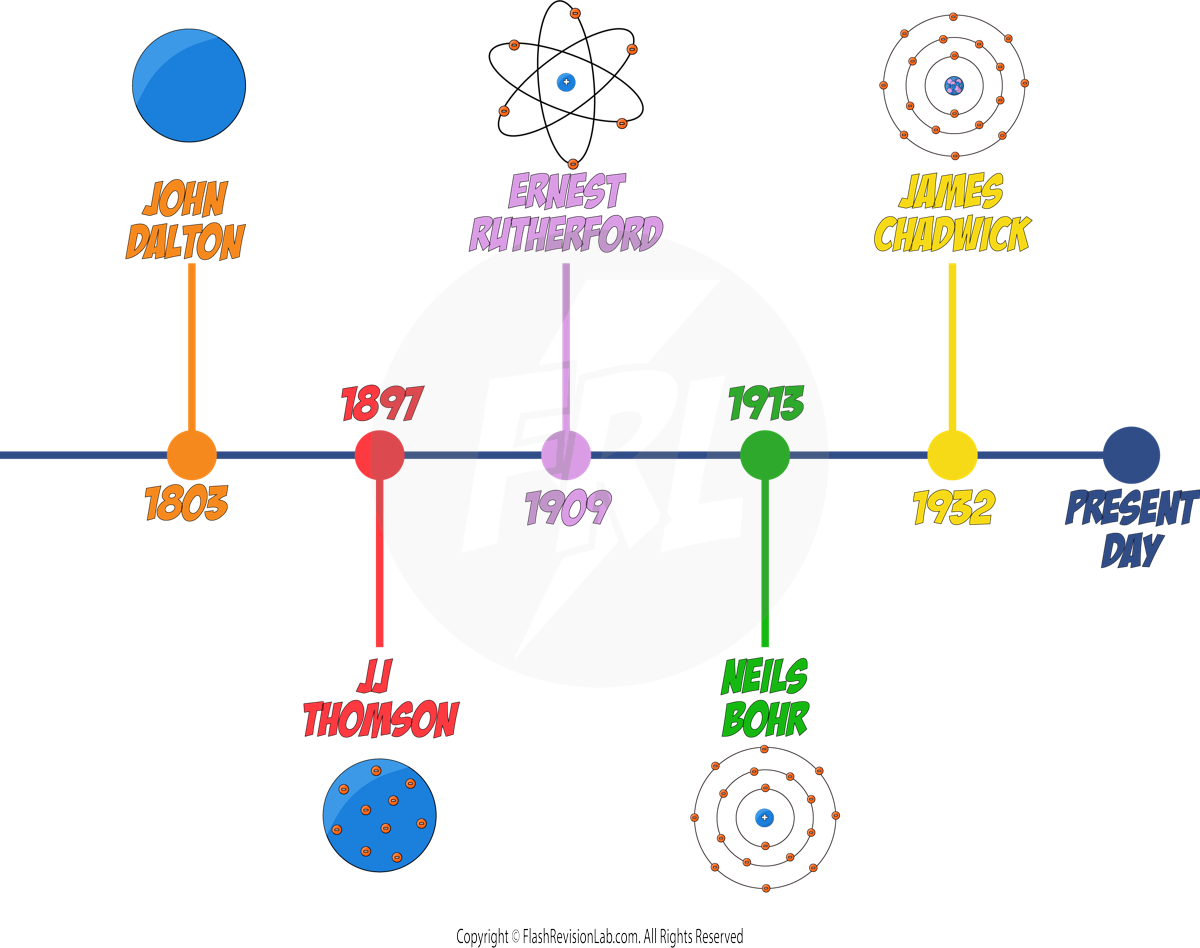
JOHN DALTON
In the early 19th century, JOHN DALTON proposed that atoms were solid SPHERES, and different spheres represented different ELEMENTS.

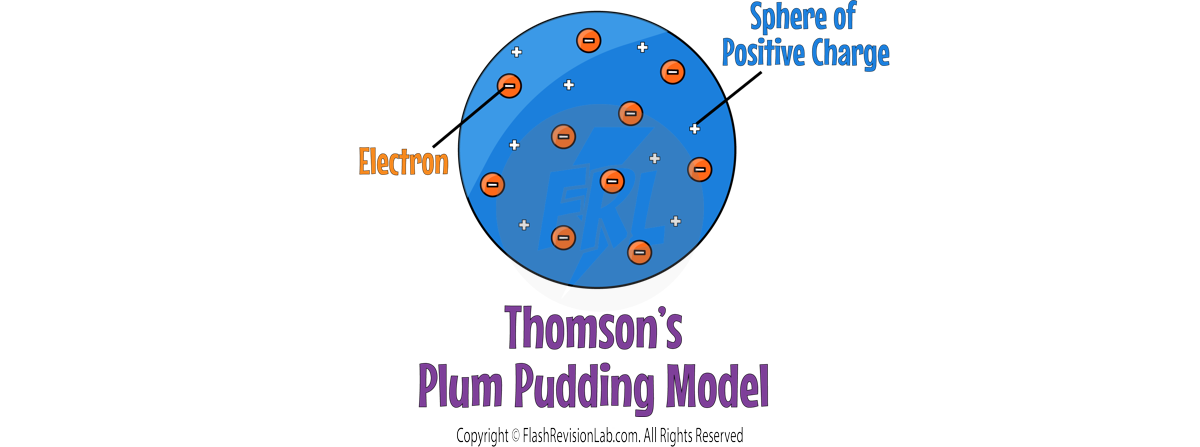
JJ THOMSON
By 1897, JJ. THOMSON had discovered ELECTRONS through experiments, leading to the "PLUM PUDDING MODEL", which showed the atom as a SPHERE of POSITIVE charge with ELECTRONS EMBEDDED within it.
ERNEST RUTHERFORD
- ERNEST RUTHERFORD and his student Ernest Marsden conducted the ALPHA PARTICLE SCATTERING experiment, which disproved the Plum Pudding model.
- During this experiment, they fired ALPHA PARTICLES at a GOLD FOIL.
- The gold foil was so thin, that they could assume that it was ONE ATOM THICK.
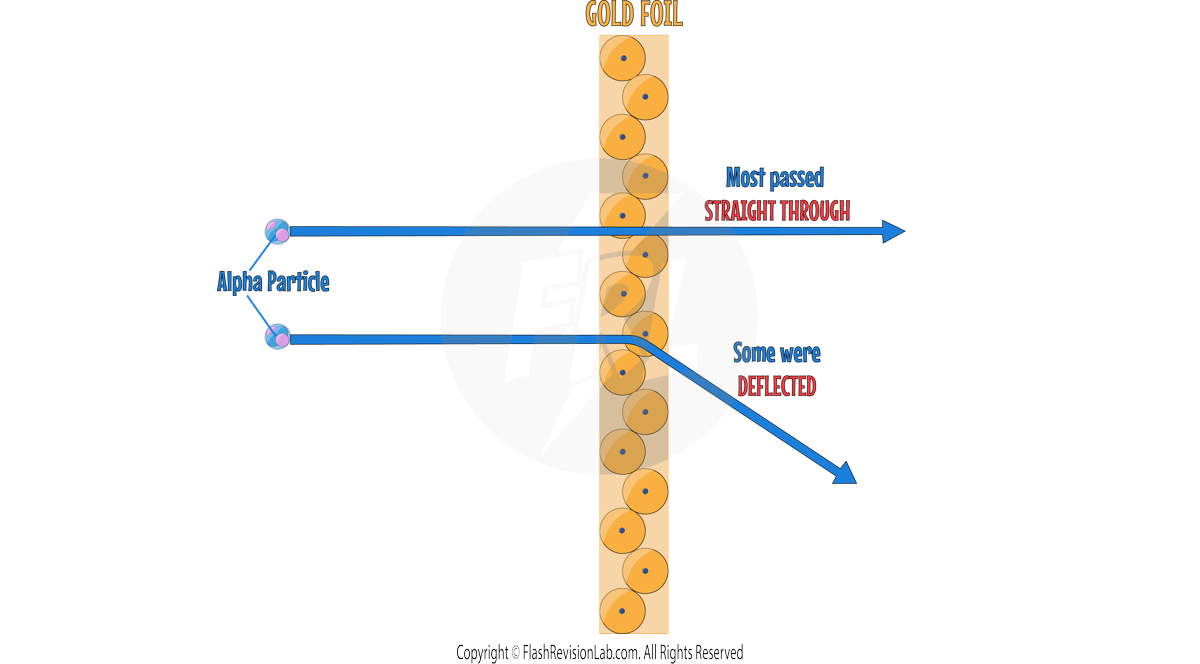
- TWO main observations were made during this experiment which led to TWO discoveries about the atom.
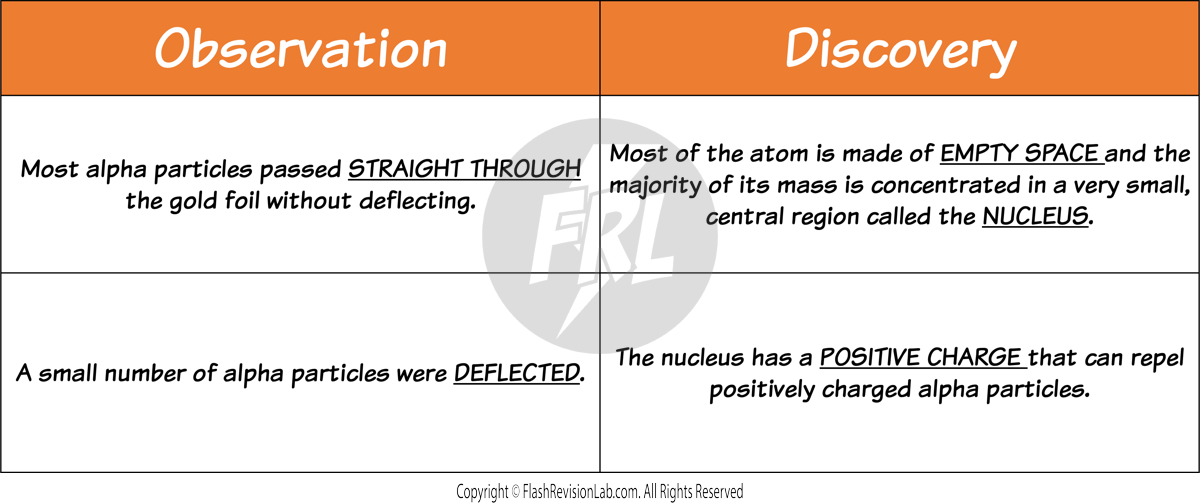
- They observed that most alpha particles passed straight through the gold foil, but some were deflected more than expected. Suggesting a small, dense, positively charged nucleus at the centre of the atom.
- The Plum Pudding model does NOT explain the results because it shows the whole atom as a ball of positive charge with NO EMPTY SPACE.
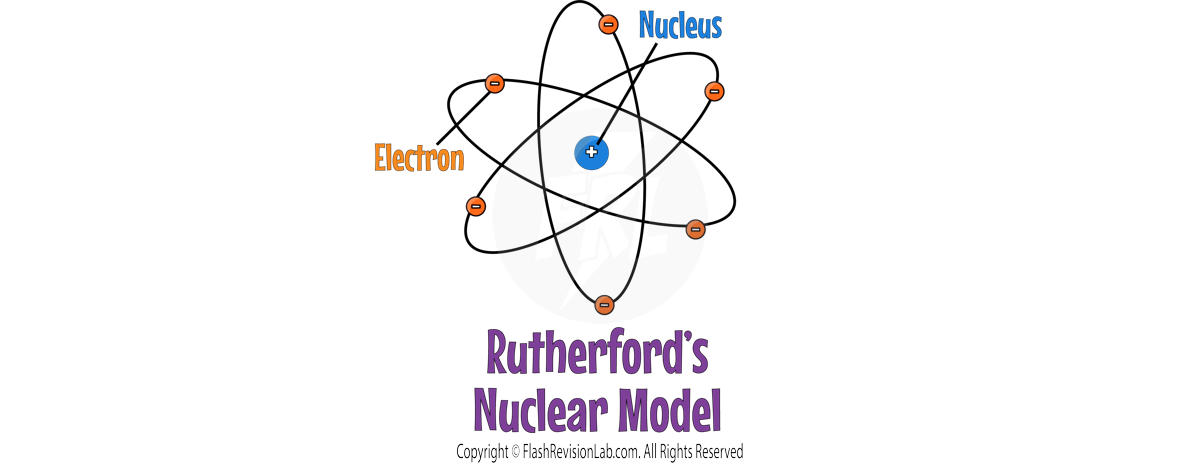
- Rutherford's findings led to the conclusion that the atom consists mostly of empty space, with a dense nucleus surrounded by a cloud of electrons.
NIELS BOHR
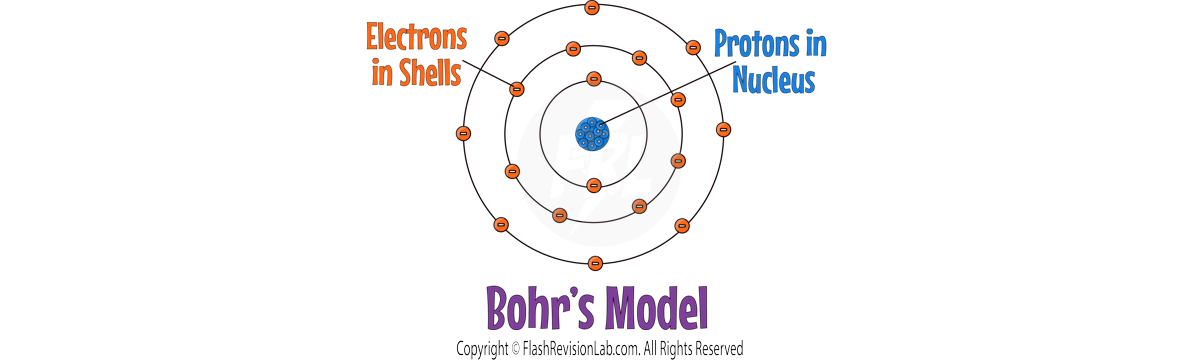
- NIELS BOHR refined the nuclear model by introducing the idea that ELECTRONS ORBIT the nucleus in fixed SHELLS.
- Bohr's model suggested that electrons could only occupy certain orbits or ENERGY LEVELS, at FIXED DISTANCES from the nucleus.
- His theoretical calculations agreed with his experimental observations which confirmed his model.
- Bohr carried out further experiments which revealed the nucleus could be SUBDIVIDED into a whole number of smaller particles, each particle having the same amount of POSITIVE charge. These were called PROTONS.
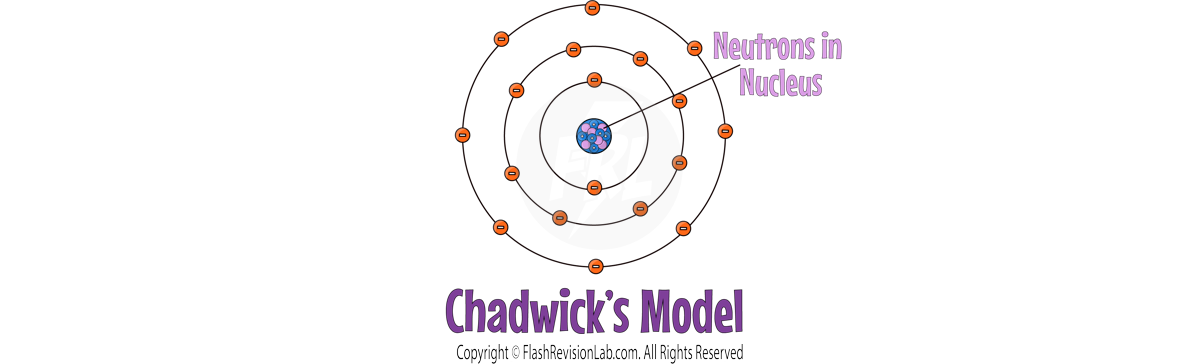
JAMES CHADWICK
- 20 years later, JAMES CHADWICK later discovered NEUTRONS, neutral particles in the nucleus, solidifying the nuclear model close to what is accepted today.
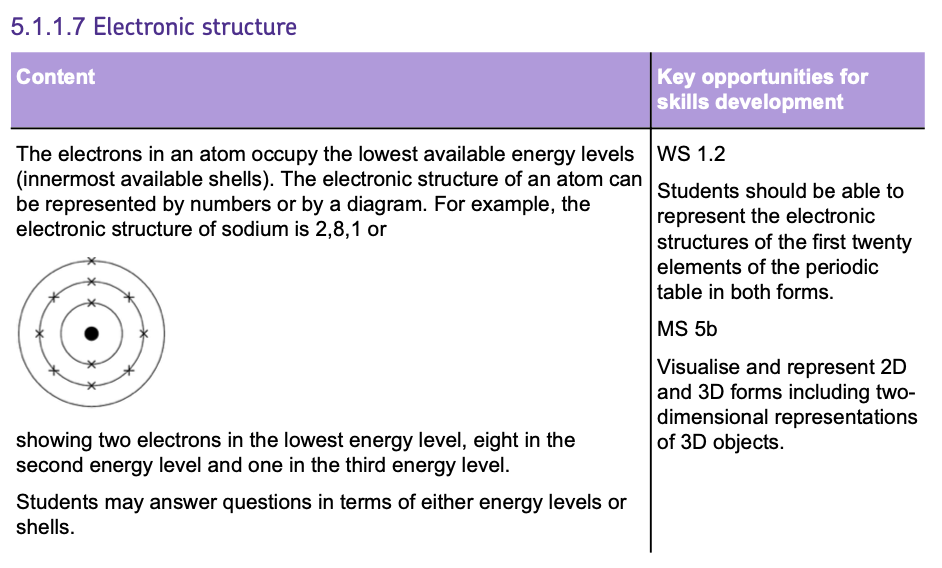
Electron Structure
- ELECTRONS are organised around the nucleus in SHELLS or energy levels, which determine the CHEMICAL PROPERTIES of an atom.
- These shells are filled from the LOWEST to the HIGHEST energy levels, with the INNERMOST shells being filled FIRST.
ELECTRON SHELL RULES
- The FIRST shell can hold up to 2 ELECTRONS.
- The SECOND and THIRD shells can each hold up to 8 ELECTRONS.
ELECTRON CONFIGURATIONS
- Electron configurations can be represented by diagrams or as numbers indicating how many electrons are in each shell.
- For example, the electronic configuration for MAGNESIUM is shown as 2, 8, 2 reflecting its distribution of electrons across the shells (2 in the first shell, 8 in the second and 2 in the third)
- The ATOMIC NUMBER of an element tells you how many protons and, by default, how many ELECTRONS the atom has if it is neutral.
- To determine an element's electronic structure, allocate electrons to the shells according to the rules, starting with the shell closest to the nucleus until you have distributed electrons equal to the atomic number.
Examples of Electronic Structures:
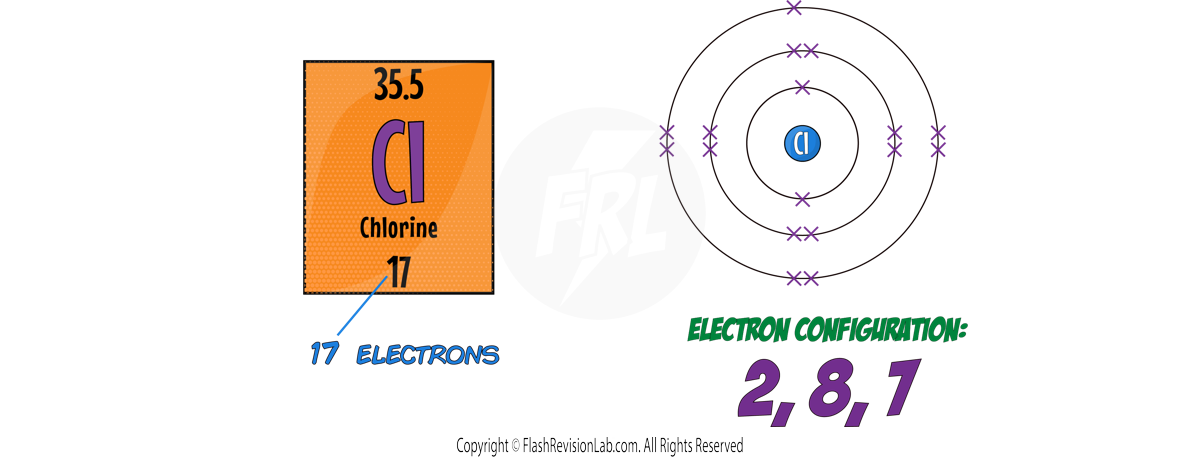
- Using the periodic table, you can see that Chlorine has an atomic number of 17, so Chlorine atoms have 17 ELECTRONS.
- The first shell will be filled with 2 ELECTRONS and the second will be filled with 8 ELECTRONS. There are 7 ELECTRONS left which all go into the THIRD shell.
- This gives the electronic structure for CHLORINE as 2, 8, 7.
The Periodic Table
Early Periodic Tables
- In the early 1800s, elements were primarily organised by ATOMIC WEIGHT and their PHYSICAL AND CHEMICAL PROPERTIES.
- ATOMIC WEIGHT was the only measurable data early scientists could use, as the concept of protons, neutrons and electrons was not yet discovered.
- This led to the creation of early periodic tables, where PROPERTIES kept repeating PERIODICALLY, hence the term PERIODIC TABLE.
- But these early periodic tables were INCOMPLETE and some elements were placed in INAPPROPRIATE GROUPS if the strict order of ATOMIC WEIGHTS was followed.
Mendeleev's Periodic Table
In 1969, DMITRI MENDELEEV, improved upon earlier tables by arranging 50 known elements by ATOMIC WEIGHT but also considered their PROPERTIES to maintain consistency within groups.
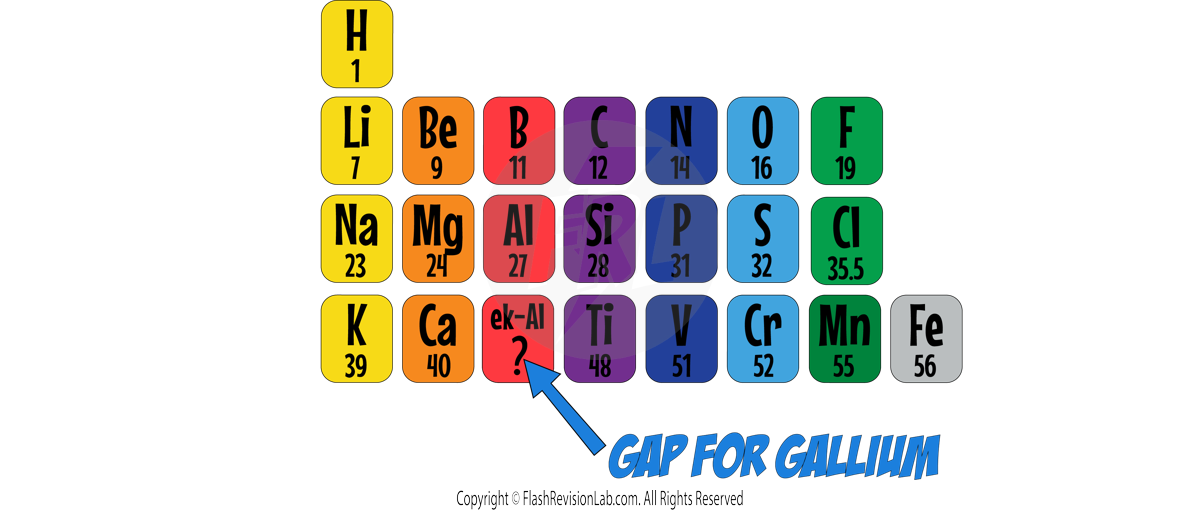
He did NOT force elements into groups that didn’t fit the patterns. Instead he made TWO CHANGES:
1. He left GAPS for elements that he thought would fit the groups but had NOT been DISCOVERED yet.
E.g. Mendeleev PREDICTED the existence and PROPERTIES of EKA-ALUMINIUM (now known as GALLIUM)
2. He CHANGED the ORDERS of some elements, even though their atomic weights did not fit this order.
E.g. Tellurium (Te) and Iodine (I). Usually, elements are lined up by their weight, and Iodine should be before Tellurium because it's lighter. But Mendeleev switched them. He put Tellurium before Iodine because its properties were more similar to the other elements in its column.
These TWO CHANGES turned out to be the right thing to do because:
1. The elements he left gaps for were discovered later and FITTED the pattern he had PREDICTED, which solidified his theories.
2. The discovery of ISOTOPES in the early 20th century confirmed Mendeleev's decision not to place elements in a strict order of atomic weight but to account for their chemical properties. This is because isotopes have different masses BUT they have the SAME CHEMICAL PROPERTIES so they occupy the SAME POSITION on the periodic table.
Eventually Mendeleev’s periodic table developed into the modern table we use today.
The Modern Periodic Table
The PERIODIC TABLE arranges about 100 ELEMENTS, based on increasing ATOMIC (PROTON) NUMBER.
It reveals repeating patterns in the properties of the elements, which is why properties recur PERIODICALLY.
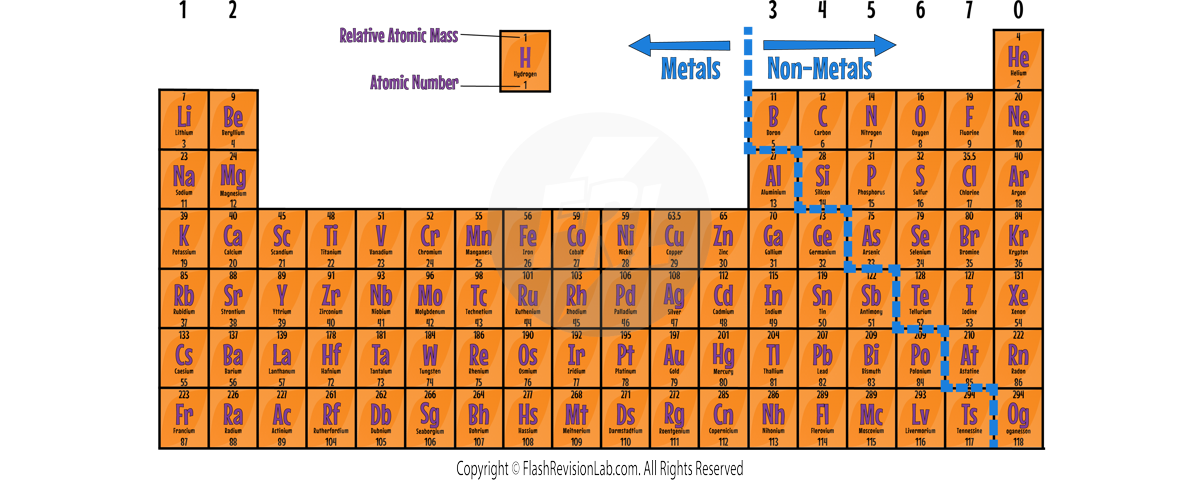
The Layout of the Periodic Table:
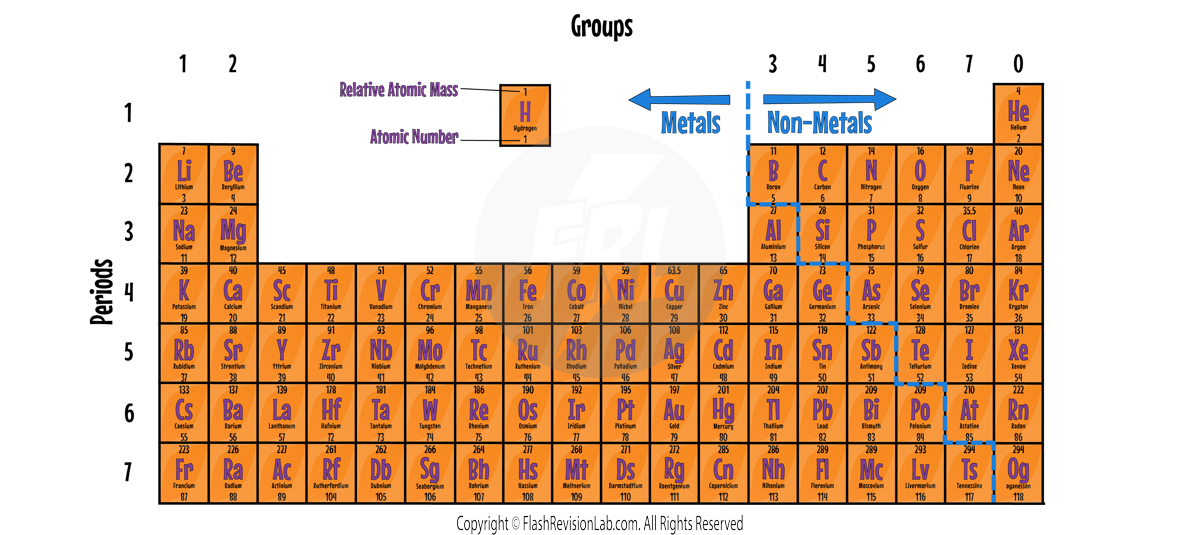
- METALS are located to the left and NON-METALS to the right, with a staircase line usually marking the division between them.
- The VERTICAL COLUMNS are called GROUPS and the HORIZONTAL ROWS are called PERIODS.
- GROUP NUMBER corresponds to the number of ELECTRONS in the outer shell. Elements in the same group have SIMILAR CHEMICAL PROPERTIES because they have the SAME number of ELECTRONS in their OUTER SHELL.
- The PERIOD NUMBER represents the NUMBER OF SHELLS an atom has.
Group and Period Trends
- Elements in the same group have similar properties and react in SIMILAR ways due to having the same number of electrons in their outer shell.
- For example: GROUP 1 elements (alkali metals) have one electron in their outer shell, making them highly reactive.
- Reactivity trends can be observed, such as Group 1 elements becoming MORE REACTIVE as you move down the group.
Predicting Element Properties
- If you know one element's properties, you can make predictions about others in the same group.
- For example, knowing the properties of Lithium (Li) helps predict the behaviour of Sodium (Na) and Potassium (K).
Metals and Non-Metals
Characteristics of Metals and Non-Metals
- METALS are elements known to form POSITIVE IONS in reactions, found mainly towards the bottom and left of the periodic table.
- The MAJORITY of elements in the periodic table are metals.
- NON-METALS do NOT form POSITIVE IONS in reactions, and are mainly found in the TOP RIGHT of the periodic table.
Reactivity:
During reactions, the atoms always try to achieve a FULL outer shell because it makes them MORE STABLE.
METALS generally LOSE ELECTRONS in order to achieve a FULL OUTER SHELL.
NON-METALS generally GAIN ELECTRONS in order to achieve a FULL OUTER SHELL.
The periodic table shows a significant link between an element's ATOMIC NUMBER and its REACTIVITY, as well as its position on the table.
Metals and Reactivity
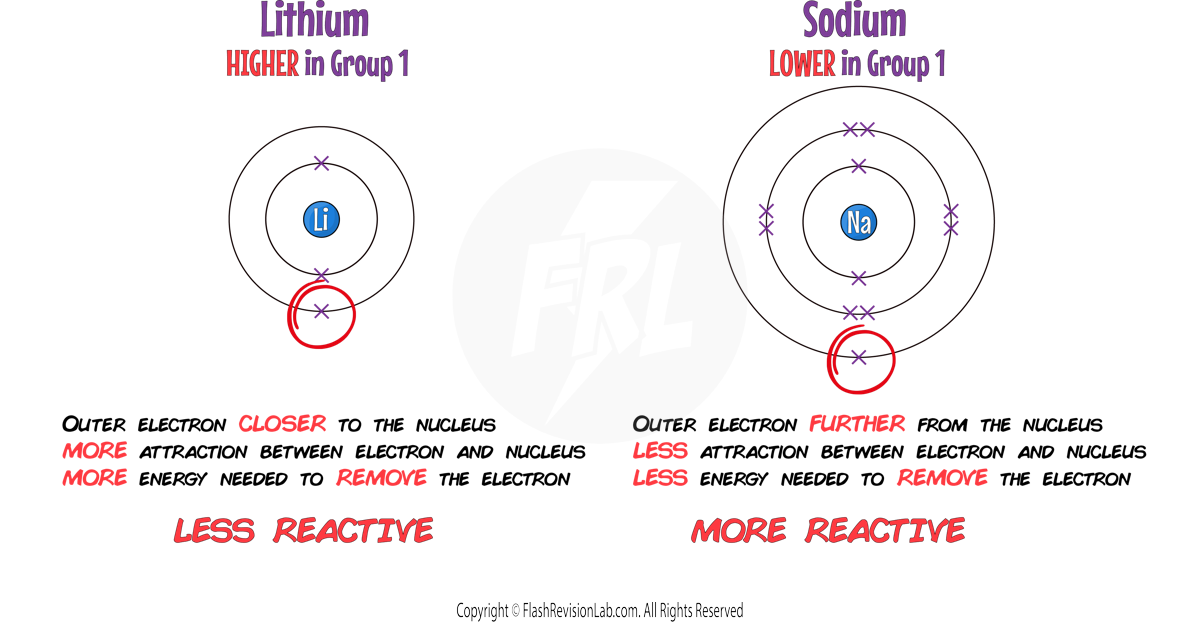
- METALS FURTHER on the LEFT side of the periodic table have fewer electrons to REMOVE from their outer shells, making them MORE REACTIVE.
- As you go DOWN a group, outer shell electrons are FURTHER AWAY from the nucleus because the atom gets BIGGER and has MORE SHELLS. This DECREASES the ATTRACTION of the outer electrons to the nucleus, which makes them MORE REACTIVE.
- REACTIVITY of METALS INCREASES down a group as it's easier for elements to react and LOSE electrons.
Non-Metals and Reactivity
- NON-METALS, found on the RIGHT-HAND SIDE of the periodic table, often have more outer electrons. They tend to GAIN or SHARE electrons to achieve a full outer shell, highlighting a distinct characteristic between metals and non-metals.
- This distinction is crucial for understanding their CHEMICAL BEHAVIOUR.
Physical Properties of Metals and Non-Metals
- METALS exhibit METALLIC BONDING, making them strong, malleable, and excellent conductors of heat and electricity.
- NON-METALS, lacking metallic bonding, often appear dull and are poor conductors of electricity, with a lower density and a wide range of physical states at room temperature.
Group 0 Elements – The Noble Gases
- NOBLE GASES, also known as GROUP 0 elements, are unique due to their COMPLETE OUTER ELECTRON SHELLS.
- This makes them INERT and COLOURLESS at room temperature.
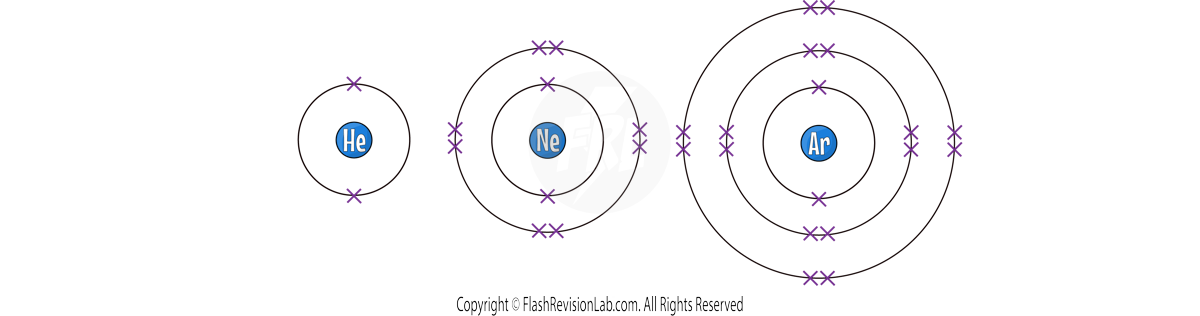
- This group includes Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn).
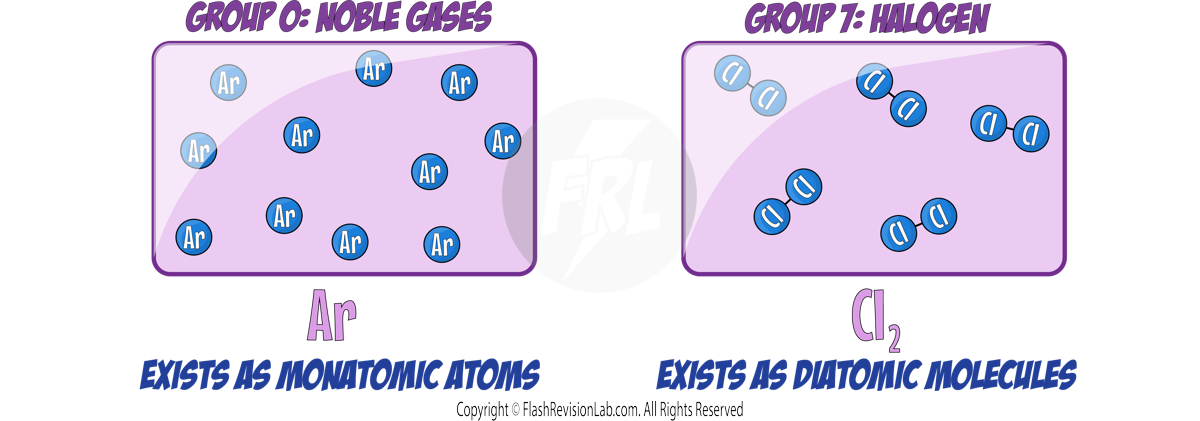
- Noble gases do NOT typically react with other elements because their outer electron shells are full, making them STABLE and UNREACTIVE.
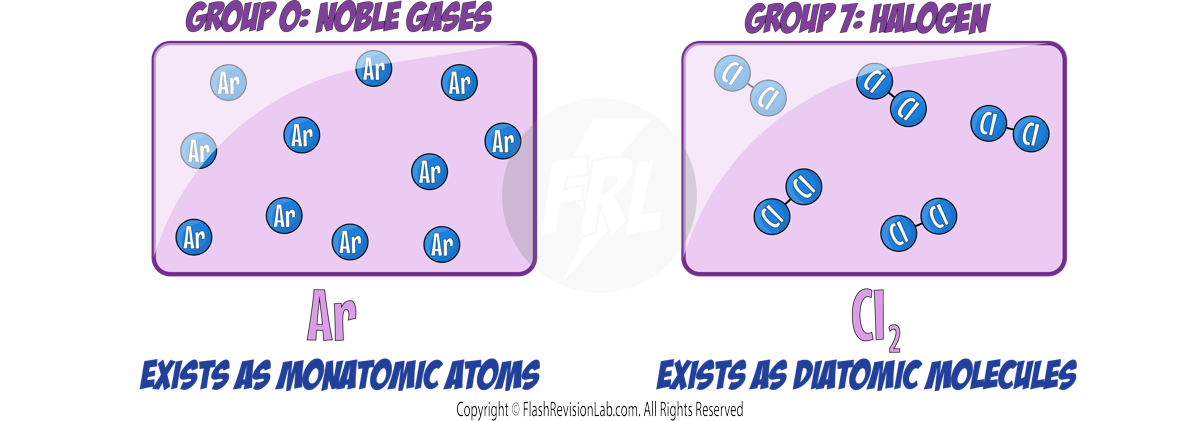
- They do NOT form molecules and exist as SINGLE MONATOMIC ATOMS. This is becuase their full outer shell makes them STABLE.
- They are NON-FLAMMABLE, meaning they do not catch fire, which is a significant safety feature in various applications.
Trends in Noble Gases
- The BOILING POINTS of Noble Gases INCREASE down the group, as RELATIVE ATOMIC MASS INCREASES.
- This rise in boiling points is due to the INCREASING number of ELECTRONS, which increases INTERMOLECULAR FORCES that need to be overcome to change state from liquid to gas.
- By knowing the trend in BOILING POINTS, you can PREDICT the boiling points of some of the Noble Gases.
Group 1 Elements - The Alkali Metals
Known as the ALKALI METALS, Group 1 elements are highly REACTIVE and are considered SOFT METALS with LOW DENSITY.
They all possess ONE ELECTRON in their OUTER SHELL, contributing to their reactivity and similar properties.
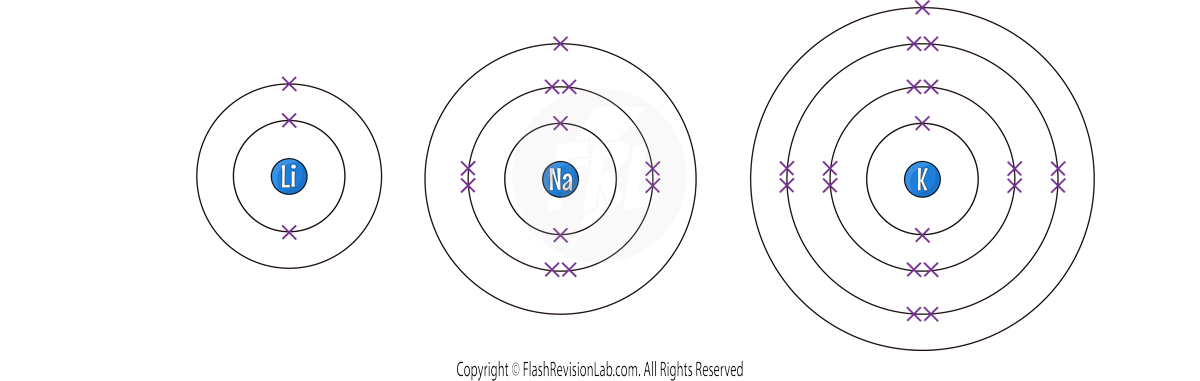
Reactivity of Alkali Metals
- The reactivity of these metals INCREASES as you go down the group. This is because the outer electron is more easily lost due to the INCREASING DISTANCE from the nucleus and a WEAKER ATTRACTION.
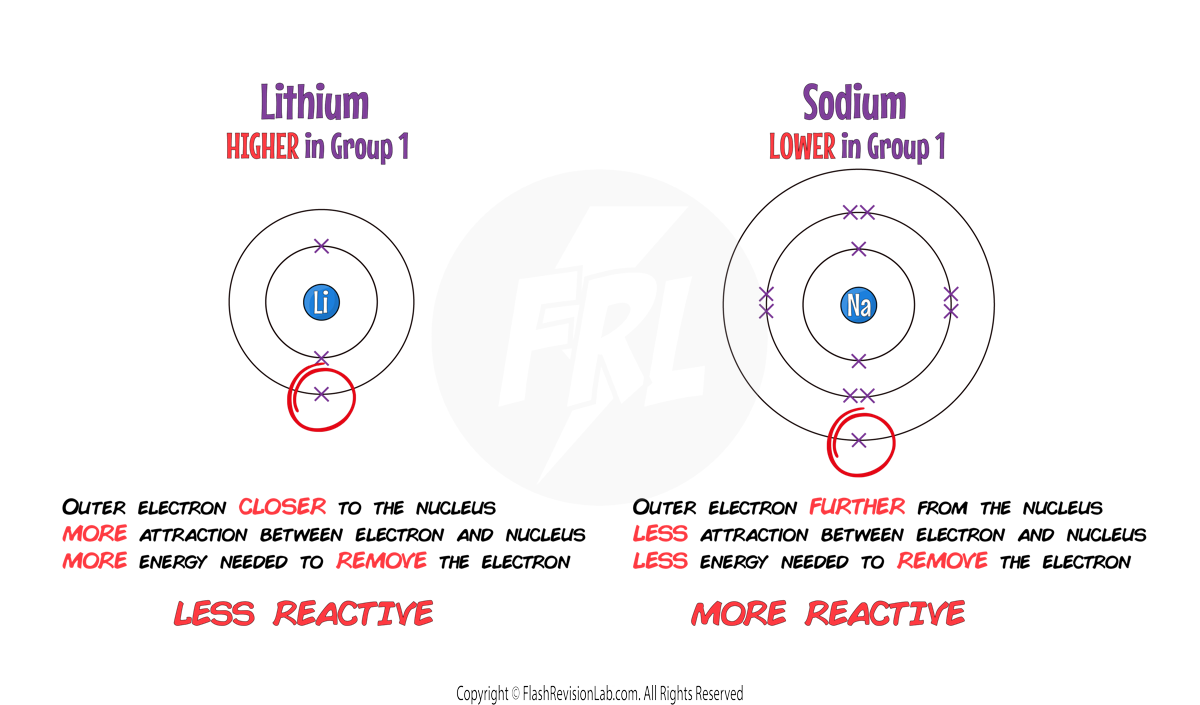
- They have LOWER MELTING AND BOILING POINTS and a HIGHER RELATIVE ATOMIC MASS as you move down the group.
- When they react, group 1 elements tend to form IONIC COMPOUNDS with non-metals, usually resulting in white solids that dissolve in water to create colourless ALKALI solutions- hence the name ALKALI METALS.
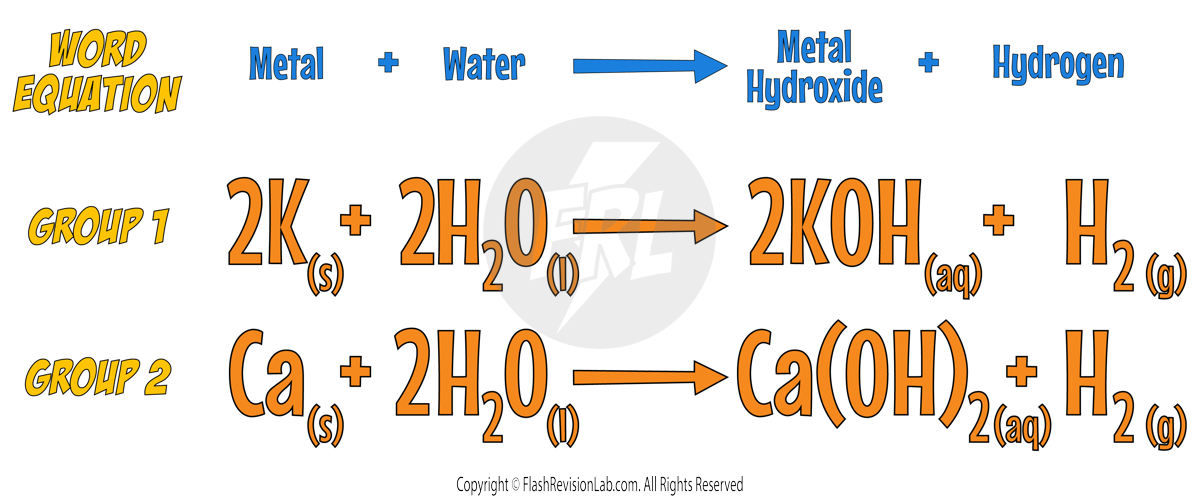
Reaction with Water
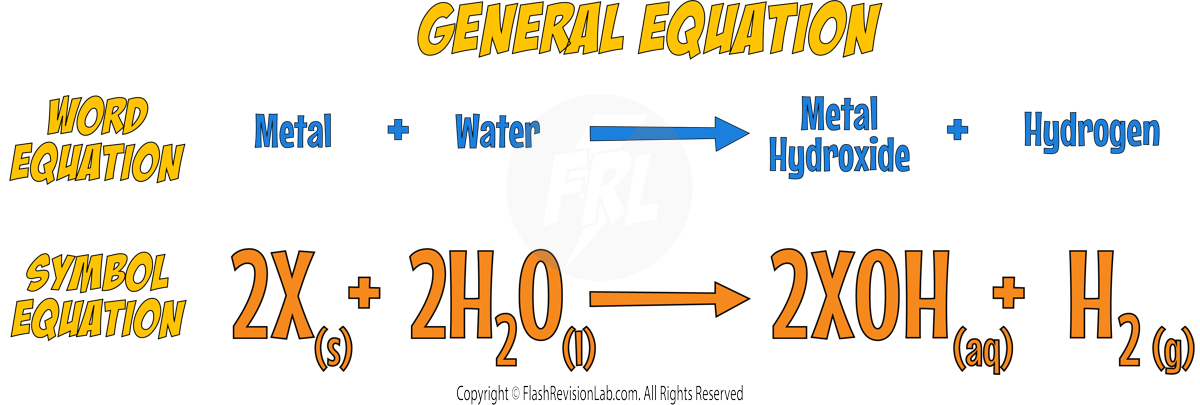
- When reacted with water, alkali metals produce Hydrogen gas and react VIGOROUSLY.
- The reactions become MORE VIOLENT reactions FURTHER DOWN the group.
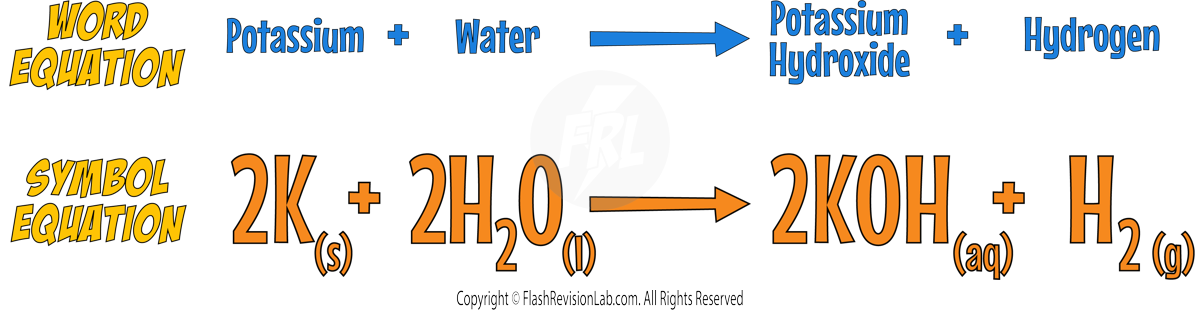
- They also form HYDROXIDES that are alkaline when dissolved in water.
- Here’s an example for POTASSIUM:
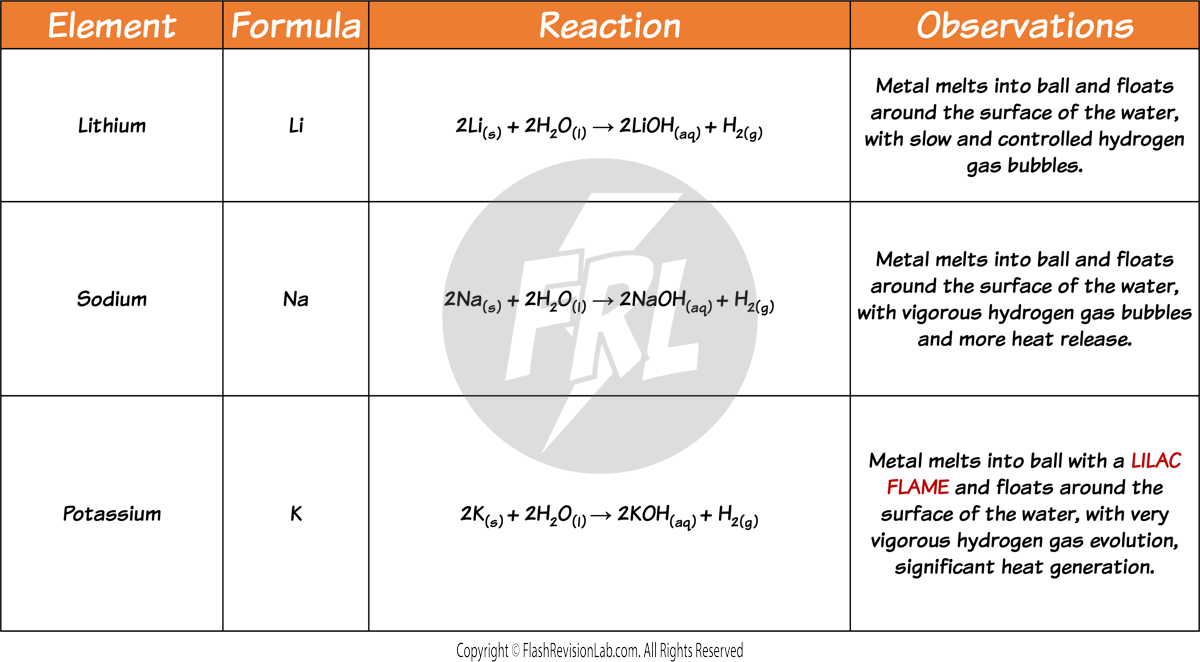
These are the OBSERVATIONS you would see for the first three ALKALI METALS:
Reaction with Chlorine
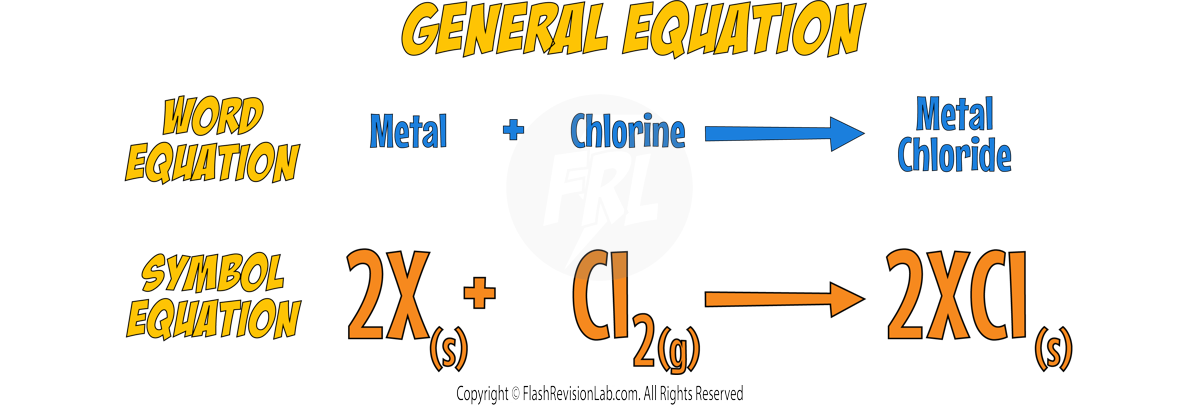
- Group 1 metals react with Chlorine to form METAL CHLORIDES, which are WHITE SALTS.
- The reactions become more VIGOROUS down the group.
- Here’s the reaction with SODIUM:
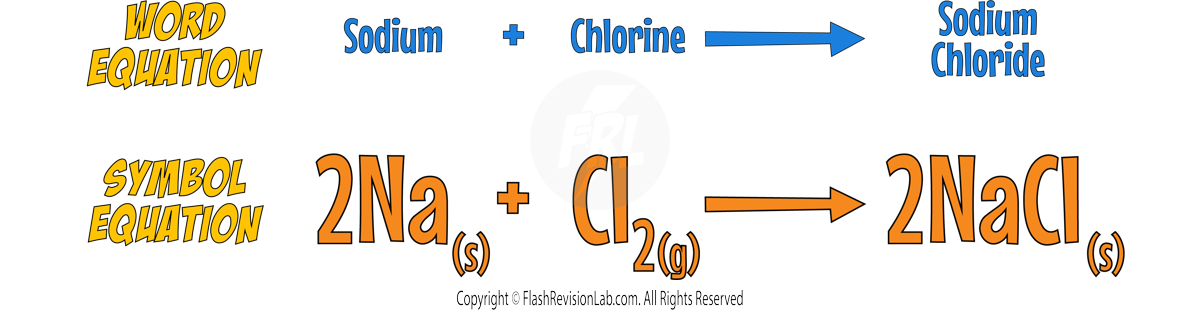
Reacting with Oxygen
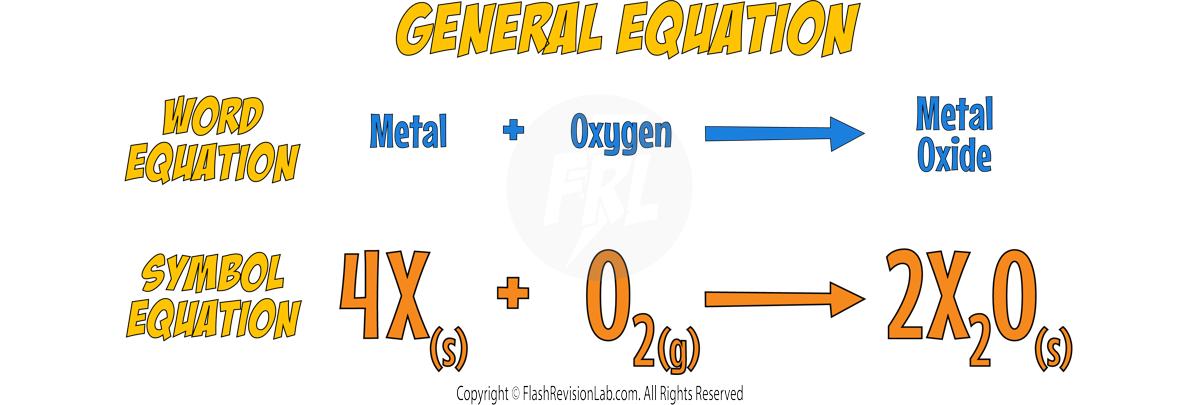
- The alkali metals react with Oxygen in the air forming METAL OXIDES, which is why the alkali metals TARNISH when exposed to the air.
- The metal oxide produced is a dull coating which covers the surface of the metal.
- Here’s the reaction with LITHIUM:
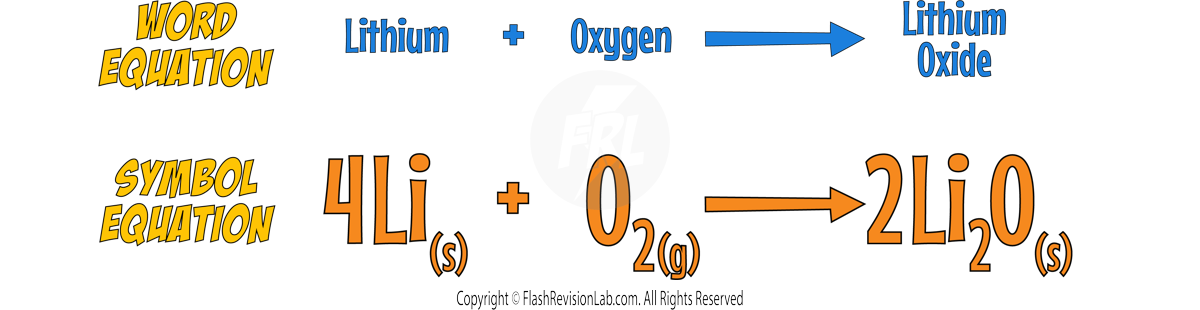
Group 7 Elements – The Halogens
- The HALOGENS are the NON-METALS found in GROUP 7.
- All halogens have SEVEN electrons in their outer shell meaning they have SIMILAR CHEMICAL properties.
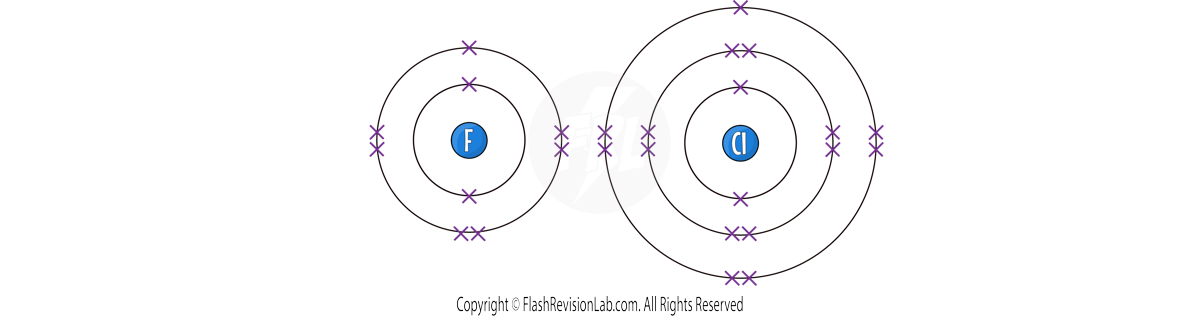
- These elements are DIATOMIC, meaning they exist naturally as molecules made of TWO ATOMS.
Trends in the Halogens
Here are the properties of the HALOGENS:
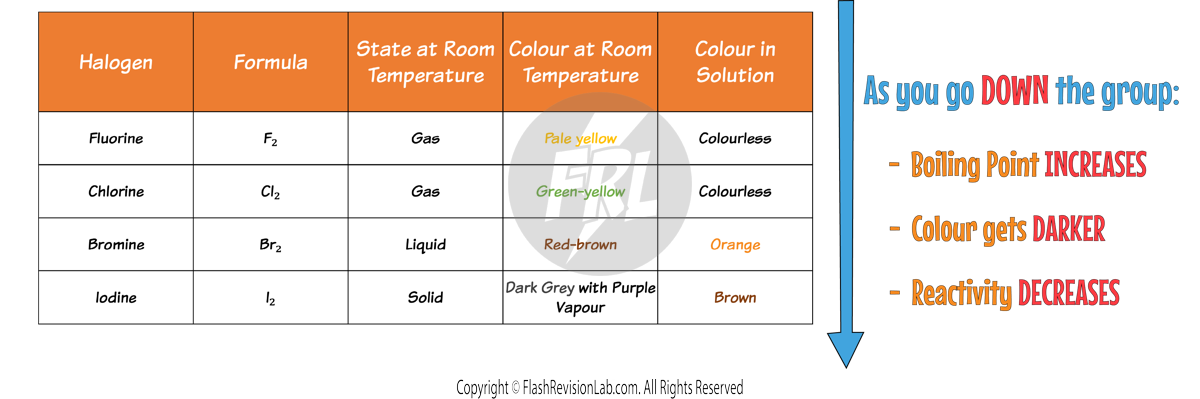
By using the trend observed, you can make PREDICTIONS about the next halogen in the group ASTATINE.
You would expect it to have a HIGHER BOILING POINT than IODINE and an even DARKER COLOUR (dark grey-black).
Explanation of the TRENDS
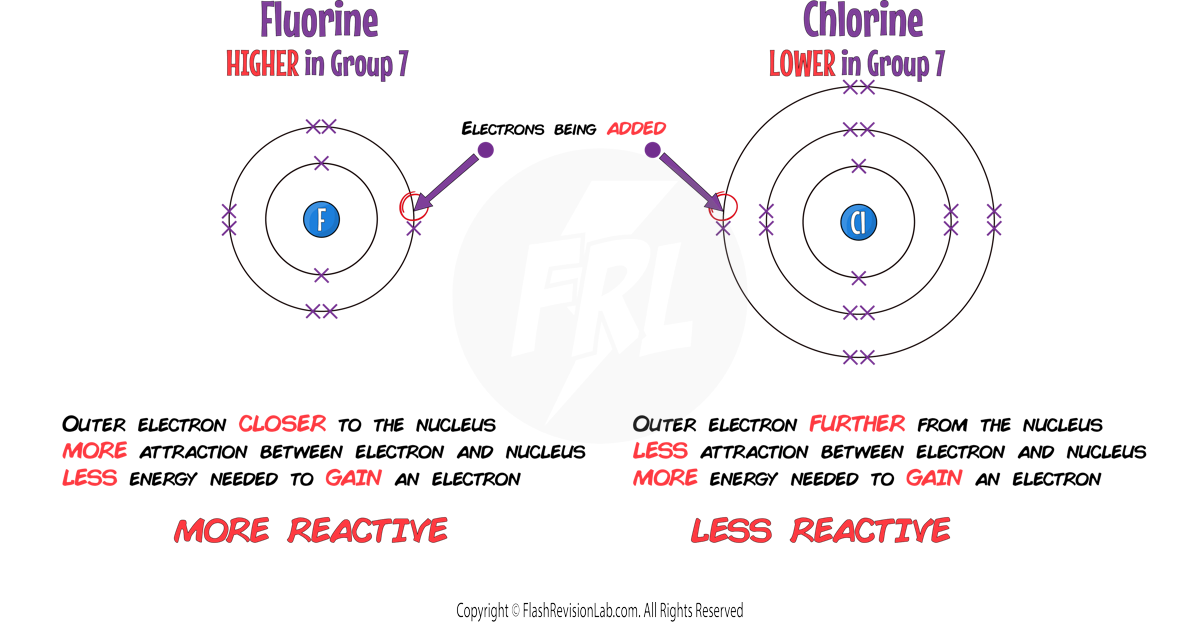
As you move DOWN GROUP 7:
1. REACTIVITY DECREASES:
When NON METALS like HALOGENS react they need to GAIN electrons.
As you go down group 7, it becomes more DIFFICULT for them to GAIN an extra electron because the number of SHELLS INCREASES and the atom gets BIGGER.
This means there is LESS ATTRACTION between the NUCLEUS and the ELECTRON being gained making it MORE DIFFICULT to add it to its outer shell.
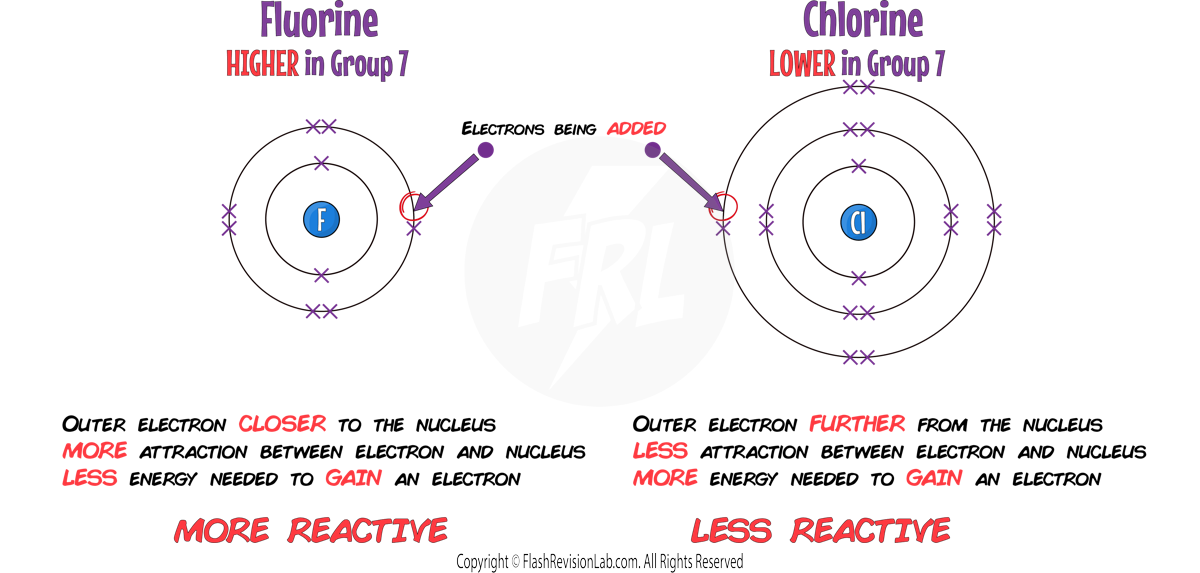
2. MELTING AND BOILING POINTS INCREASE:
The number of ELECTRONS in the molecules INCREASES down the group, meaning the INTERMOLECULAR FORCES between them get STRONGER.
This means MORE ENERGY is needed to break the bonds.
Chemical Properties
- When halogens react with NON-METALS, they form SIMPLE MOLECULAR compounds with COVALENT BONDS.
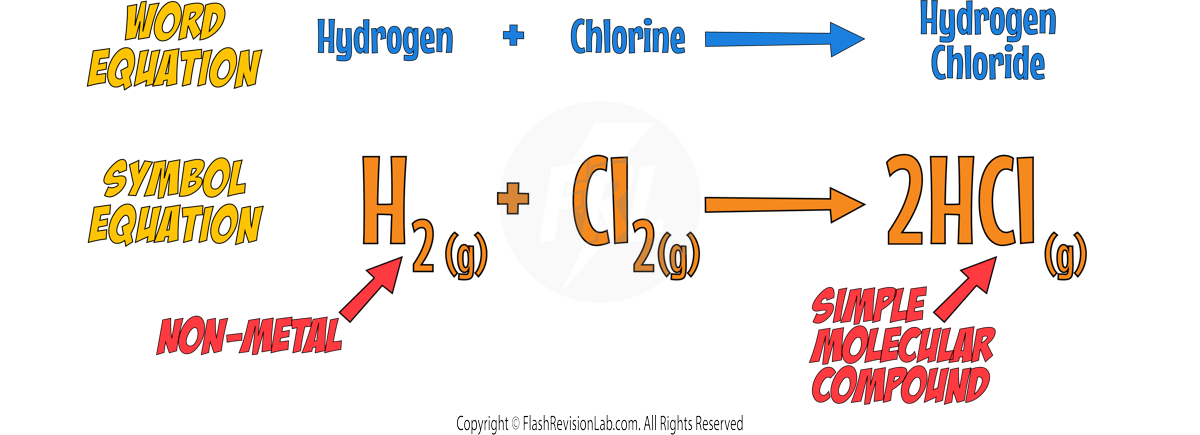
- When halogens react with METALS, they form IONIC COMPOUNDS with IONIC BONDS.
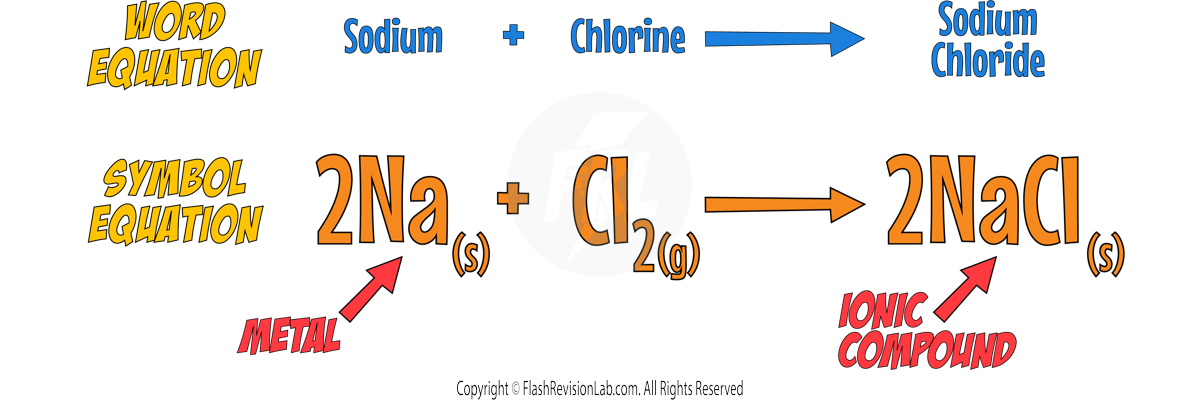
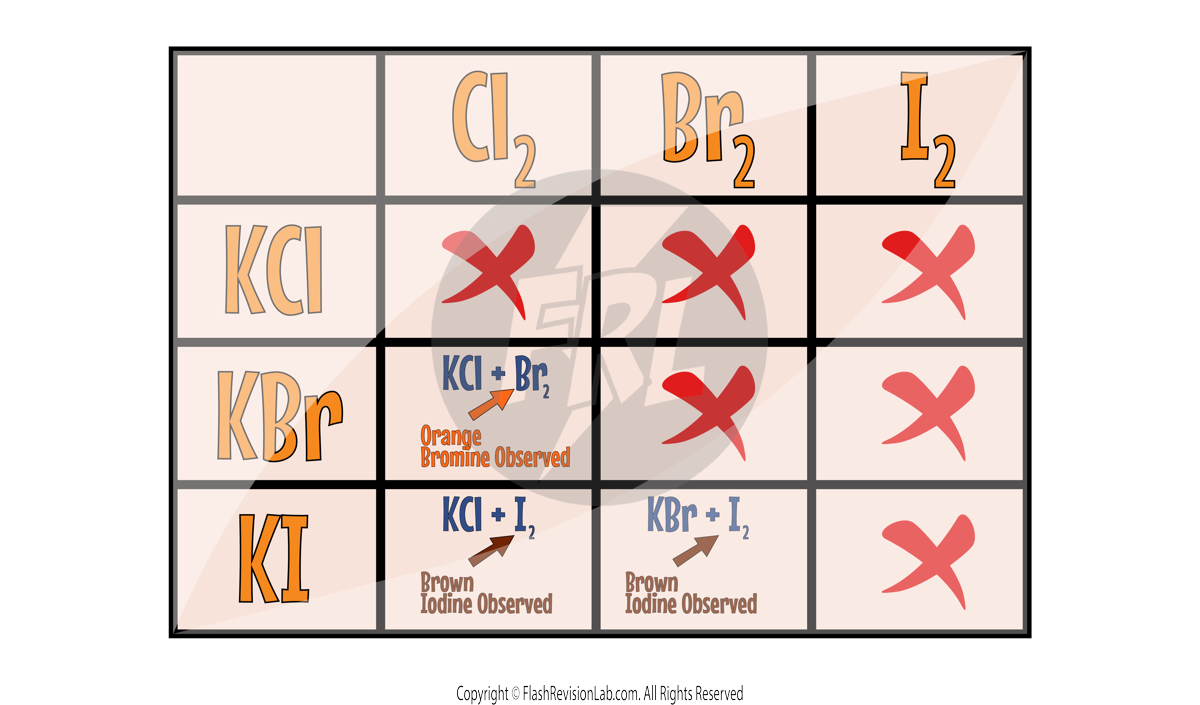
Displacement Reactions
- HALOGENS undergo DISPLACEMENT REACTIONS where a MORE REACTIVE halogen REPLACES a LESS REACTIVE halogen in a compound.
- For example, Chlorine can displace Bromine in Potassium Bromide:
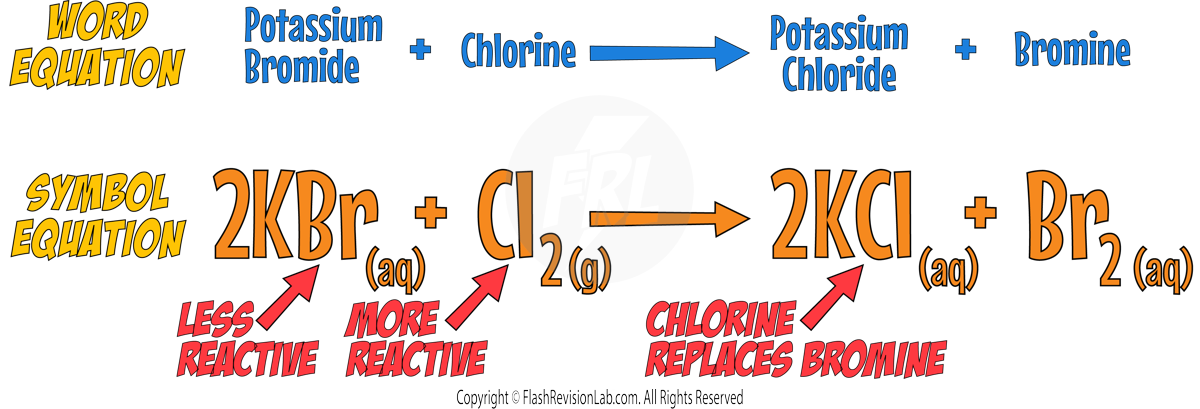
- If the halogen is LESS REACTIVE, the displacement reaction will NOT occur.
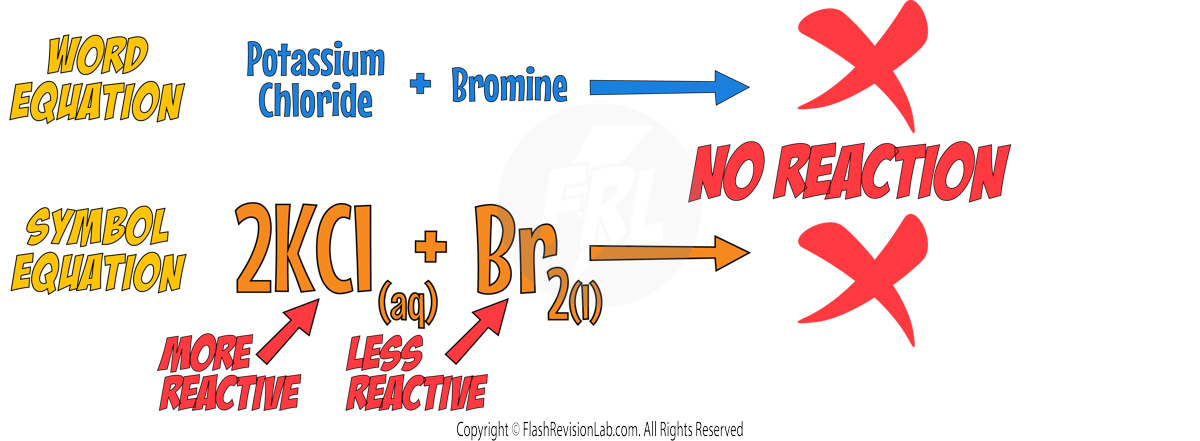
Here's a list with all the possible DISPLACEMENT reactions:
Ionic Bonding
UNDERSTANDING IONS
-
IONS are atoms or groups of atoms that have LOST OR GAINED ELECTRONS and have a CHARGE.
-
Metals LOSE ELECTRONS to form POSITIVE IONS or CATIONS.
- Non-metals GAIN ELECTRONS to form NEGATIVE IONS or ANIONS.
How Ions Form
Ions form when electrons are TRANSFERRED between atoms to achieve a FULL OUTER SHELL. This makes them MORE STABLE.
Let’s look at a SODIUM atom as an example:
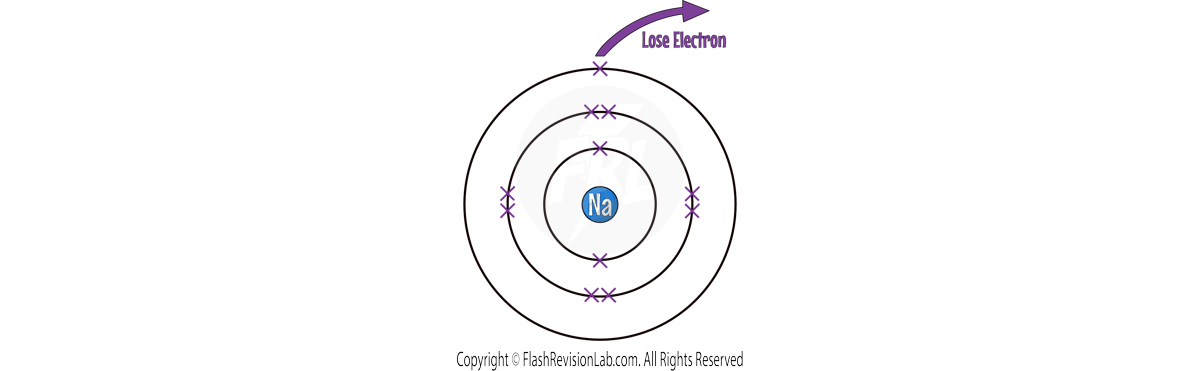
There is ONE electron in its outer shell, so the easiest way it can achieve a FULL OUTER SHELL is by LOSING 1 electron.
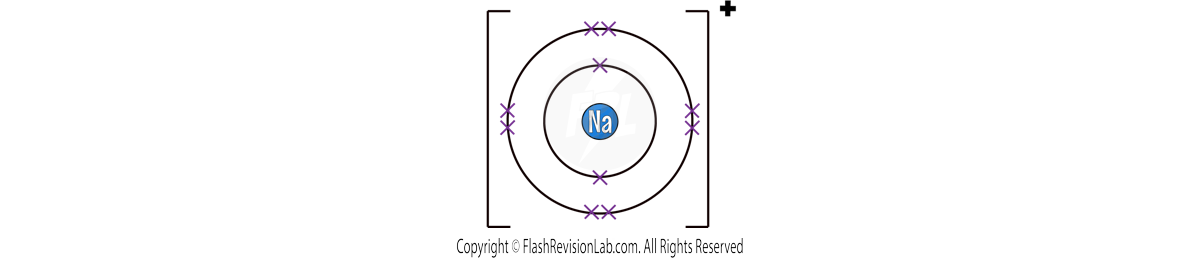
Once it has lost the electron, it becomes an ION.
To show this, you draw its structure within SQUARE BRACKETS and show its CHARGE on the top right.
It becomes POSITIVE because it has LOST a NEGATIVELY charged electron.
Now let’s look at an OXYGEN atom as an example:
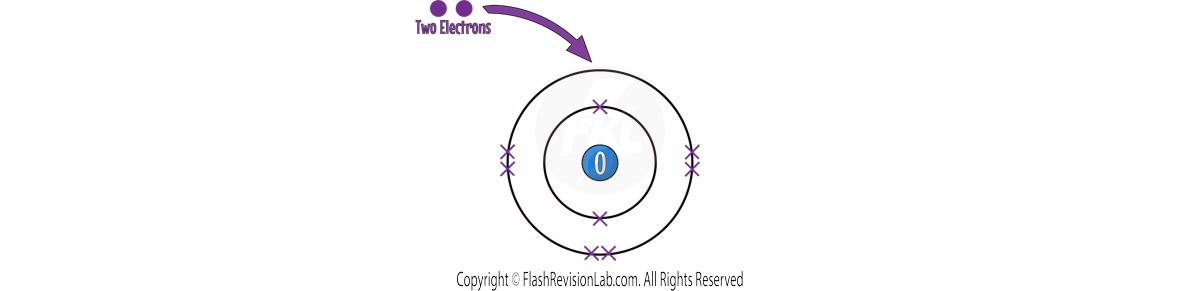
This time, the atom has SIX outer electrons, so it needs to GAIN TWO electrons to achieve a FULL OUTER SHELL.
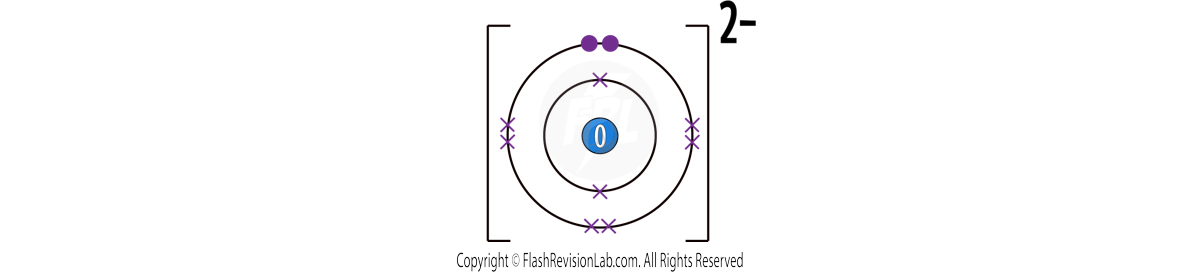
This time the extra electrons have made the atom turn into a NEGATIVE ion.
It has a charge of -2 as TWO electrons were added.
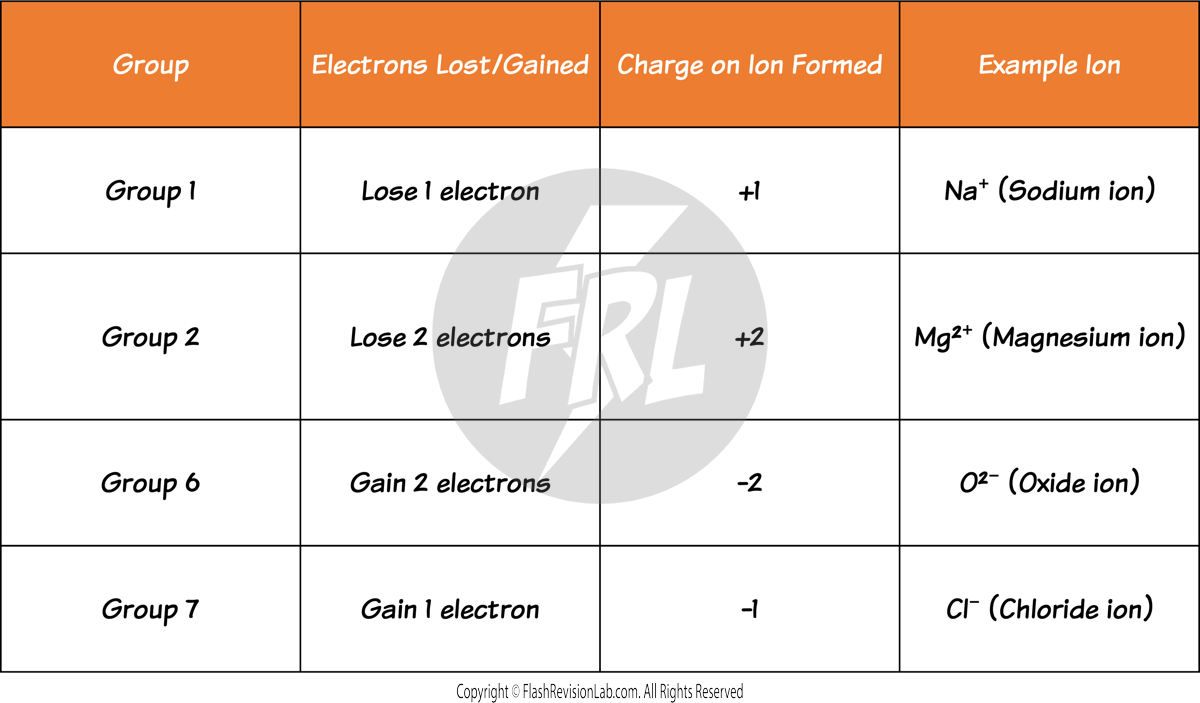
You can predict what type of ion an atom will form by knowing the GROUP of the element:
IONIC BONDING
- This type of bonding occurs between a METAL and a NON-METAL.
- IONIC BONDING involves the TRANSFER of electrons FROM a METAL atom TO a NON-METAL atom.
- The METAL atom LOSES electrons to become a POSITIVELY charged ion, while the NON-METAL atom GAINS those electrons to become a NEGATIVELY charged ion.
- These ions are then STRONGLY attracted to each other by ELECTROSTATIC forces, creating an IONIC BOND.
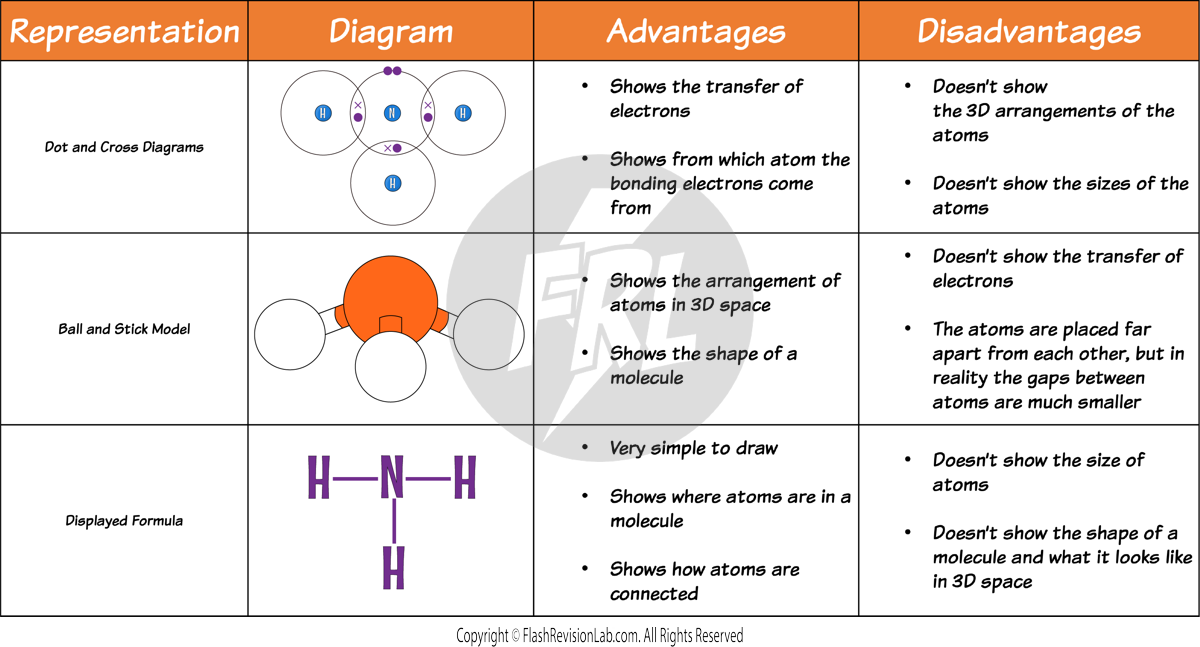
DOT AND CROSS DIAGRAMS
DOT AND CROSS DIAGRAMS represent the ARRANGEMENT of electrons in atoms or ions where each electron is symbolised by a dot or a cross. These diagrams show which atom the electrons originally came from.
EXAMPLE 1 - Let’s look at LITHIUM FLUORIDE:
Lithium has ONE outer shell electron, and Fluorine has SEVEN.
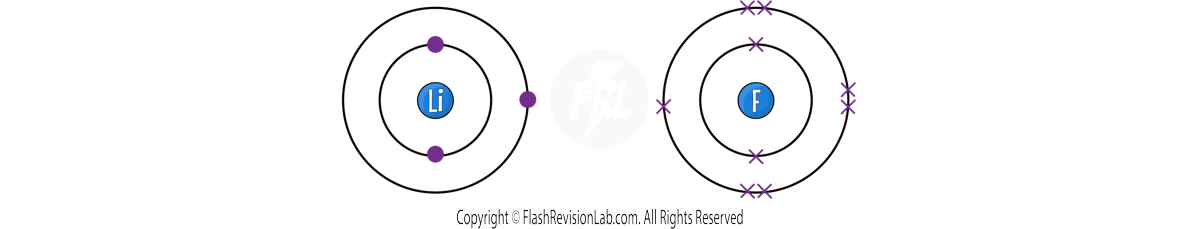
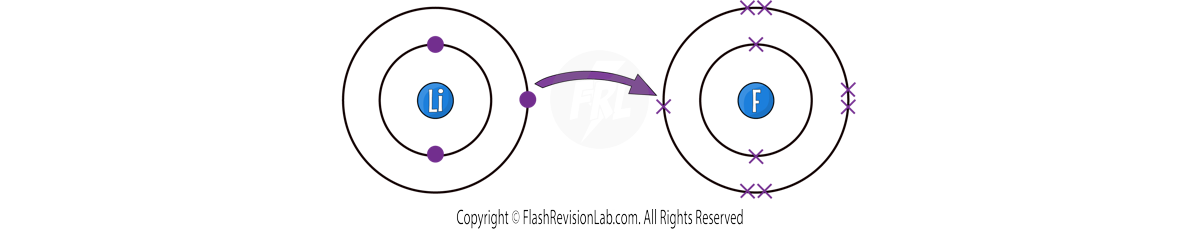
The best way for BOTH atoms to get a full outer shell is for LITHIUM to TRANSFER ONE electron to FLUORINE.
This gives BOTH atoms a FULL OUTER SHELL and STABILITY.
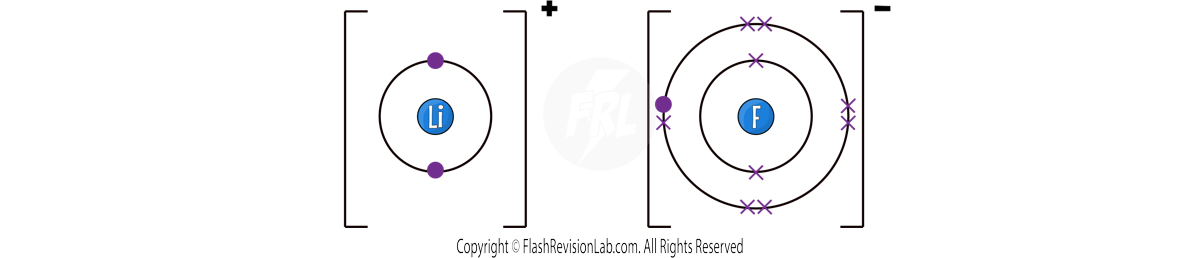
As Lithium LOSES ONE electron is gets a +1 charge and Fluorine GAINS ONE electron it gets a -1 charge.
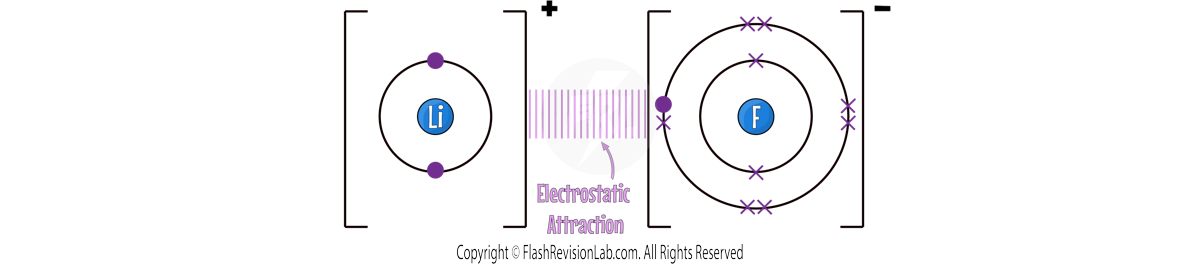
Both ions are OPPOSITELY CHARGED and they have ELECTROSTATIC FORCES of ATTRACTION holding them together. This is known as the IONIC BOND.
The Li+ and F- join to give the final formula for Lithium Fluoride as LiF.
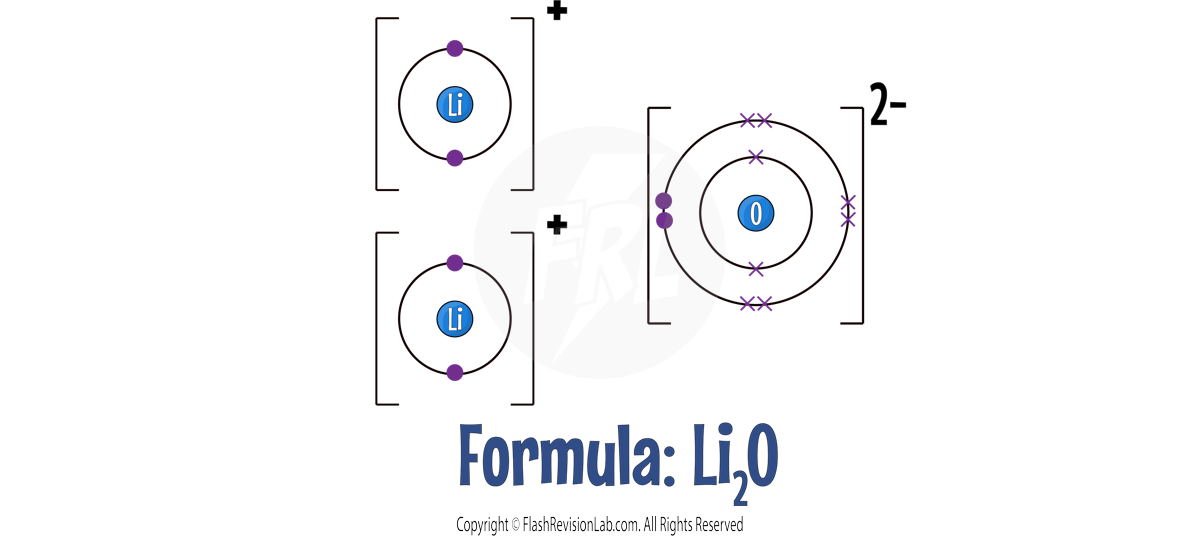
EXAMPLE 2 - Let's look at a more difficult example for LITHIUM OXIDE:
Lithium has ONE outer shell electron, but Oxygen has SIX.
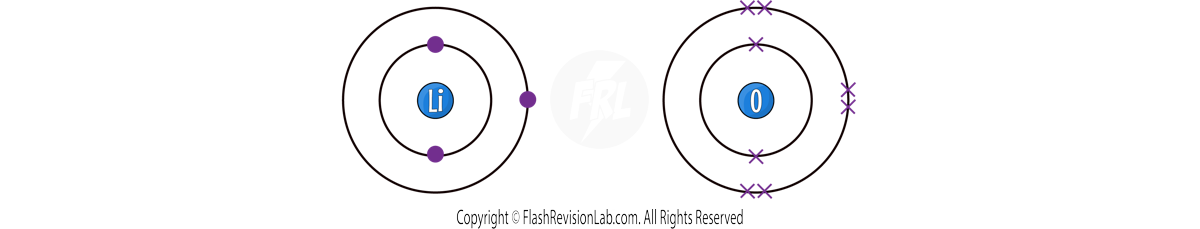
This time Lithium needs to LOSE ONE electron, but Oxygen needs to GAIN TWO. This means you would need ANOTHER Lithium atom to provide a SECOND electron to Oxygen.
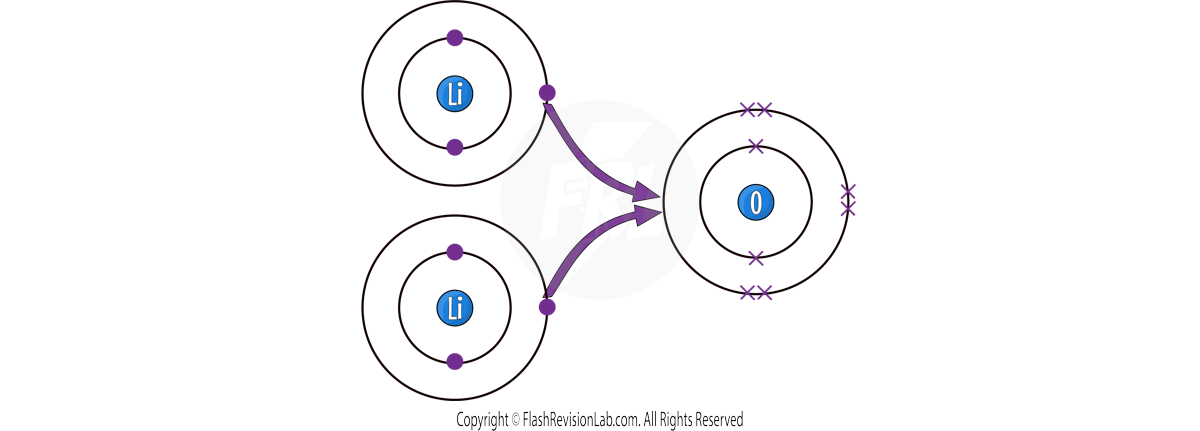
Now they are STABLE with FULL OUTER SHELLS.
The Li+ and O2- join to give the final formula for Lithium Oxide as LiO.
Ionic Compounds
Metals and Non-Metals that combine with IONIC BONDING form IONIC COMPOUNDS.
Ionic compounds are known for their GIANT IONIC LATTICE structure. This regular lattice consists of a 3D arrangement where ions are held together by strong ELECTROSTATIC FORCES of attraction in ALL DIRECTIONS.
Key Properties of Ionic Compounds
1. HIGH MELTING AND BOILING POINTS:
- There are STRONG ELECTROSTATIC FORCES of attraction between the OPPOSITELY CHARGED ions which require a LARGE amount of ENERGY to overcome.
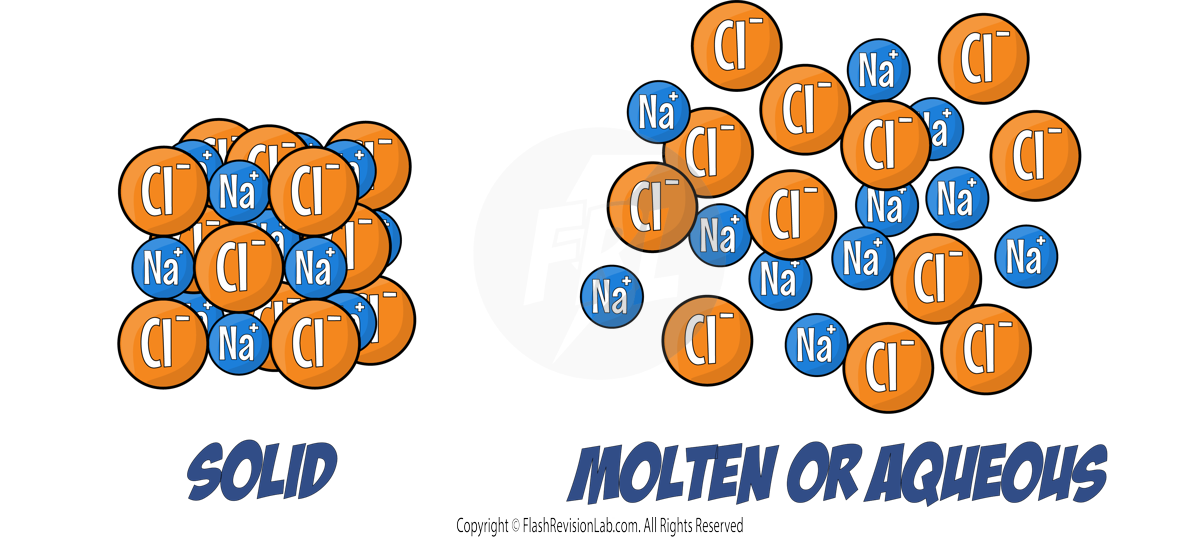
2. ELECTRICAL CONDUCTIVITY when MOLTEN or AQUEOUS:
- MOLTEN means MELTED to liquid.
- AQUEOUS means DISSOLVED in water to make a SOLUTION.
- When ionic compounds are MOLTEN or AQUEOUS, the IONS are FREE TO MOVE, meaning they can CARRY A CHARGE and conduct electricity.
3. ELECTRICAL CONDUCTIVITY when SOLID:
- IONS in the solid are FIXED meaning the IONS are NOT free to move, so they CAN’T carry a charge and conduct electricity.
4. SOLUBILITY:
- Many ionic compounds CAN DISSOLVE in water, allowing the ions to separate and move freely.
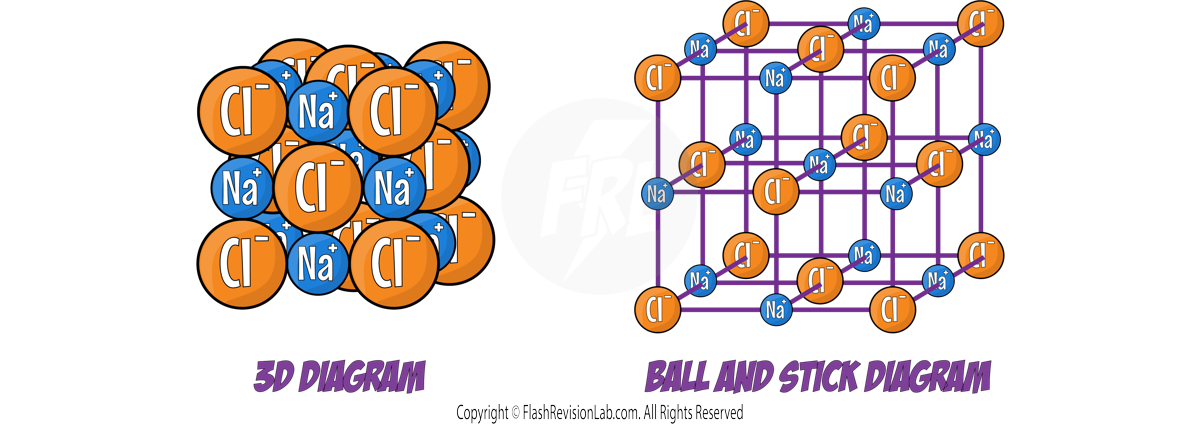
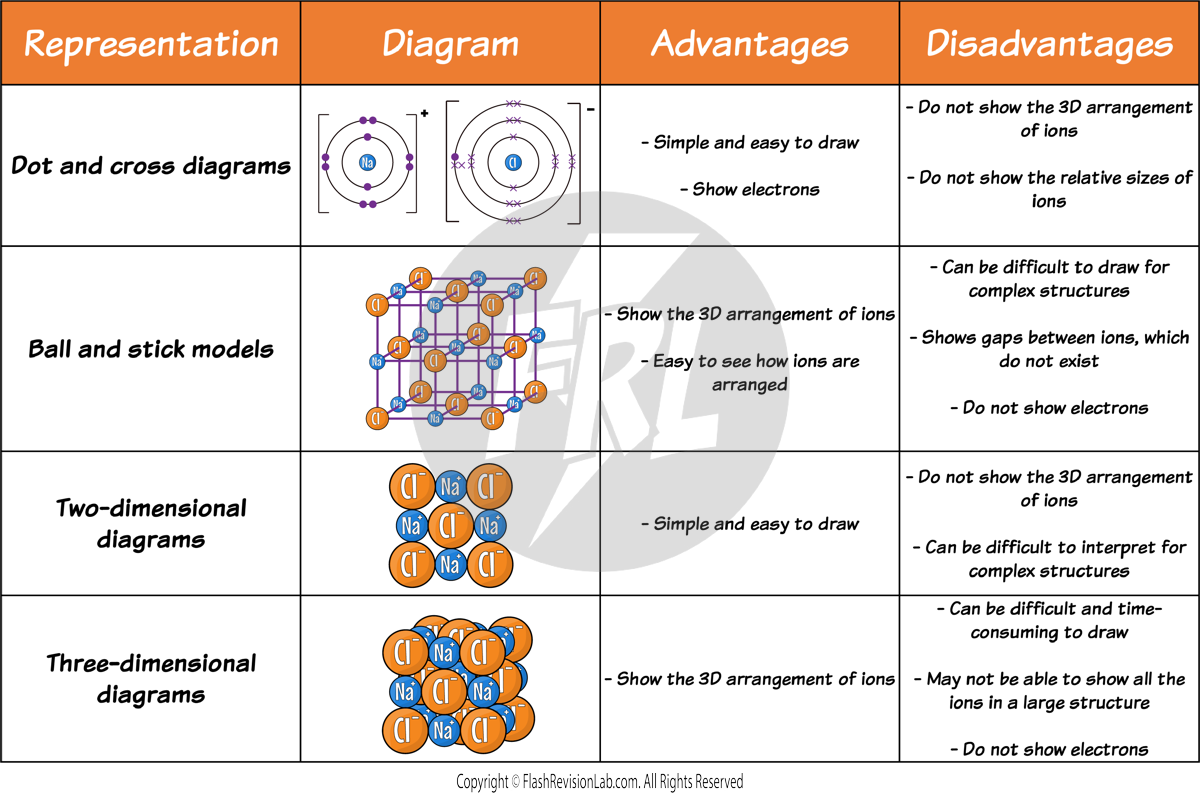
Diagrams for Ionic Compounds
There are many different ways to represent the structure of IONIC COMPOUNDS, each type of diagram has their own advantages and disadvantages:
Determining the Formula of an Ionic Compound
You can figure out the formula of an ionic compound by using its DIAGRAM or by looking at the CHARGES of the elements.
1. Use DOT AND CROSS DIAGRAMS or 3D diagrams to identify the ratio of ions in a compound.
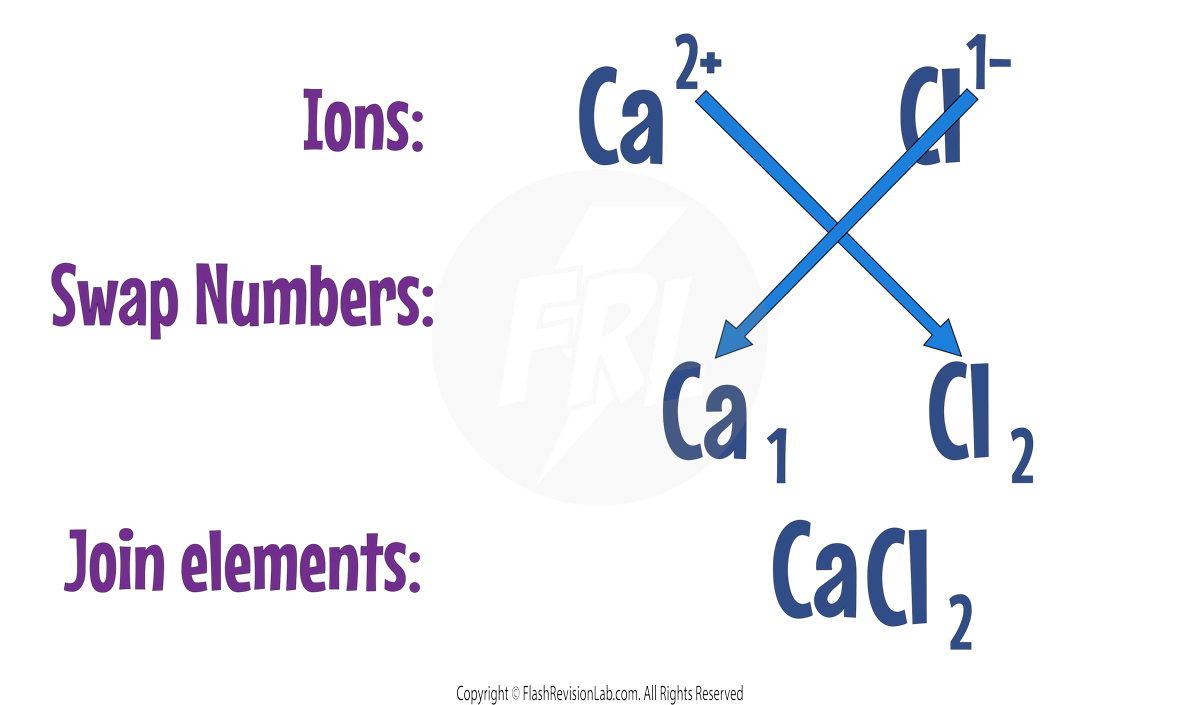
2. BALANCE THE CHARGES of ions to ensure the overall charge of the compound is zero.
- You can do this by SWAPPING the numbers of their charges.
- Let’s look at Calcium Chloride as an example:
Calcium has a charge of +2 because it is in group 2.
Chlorine has a charge of -1 because it is in group 7.
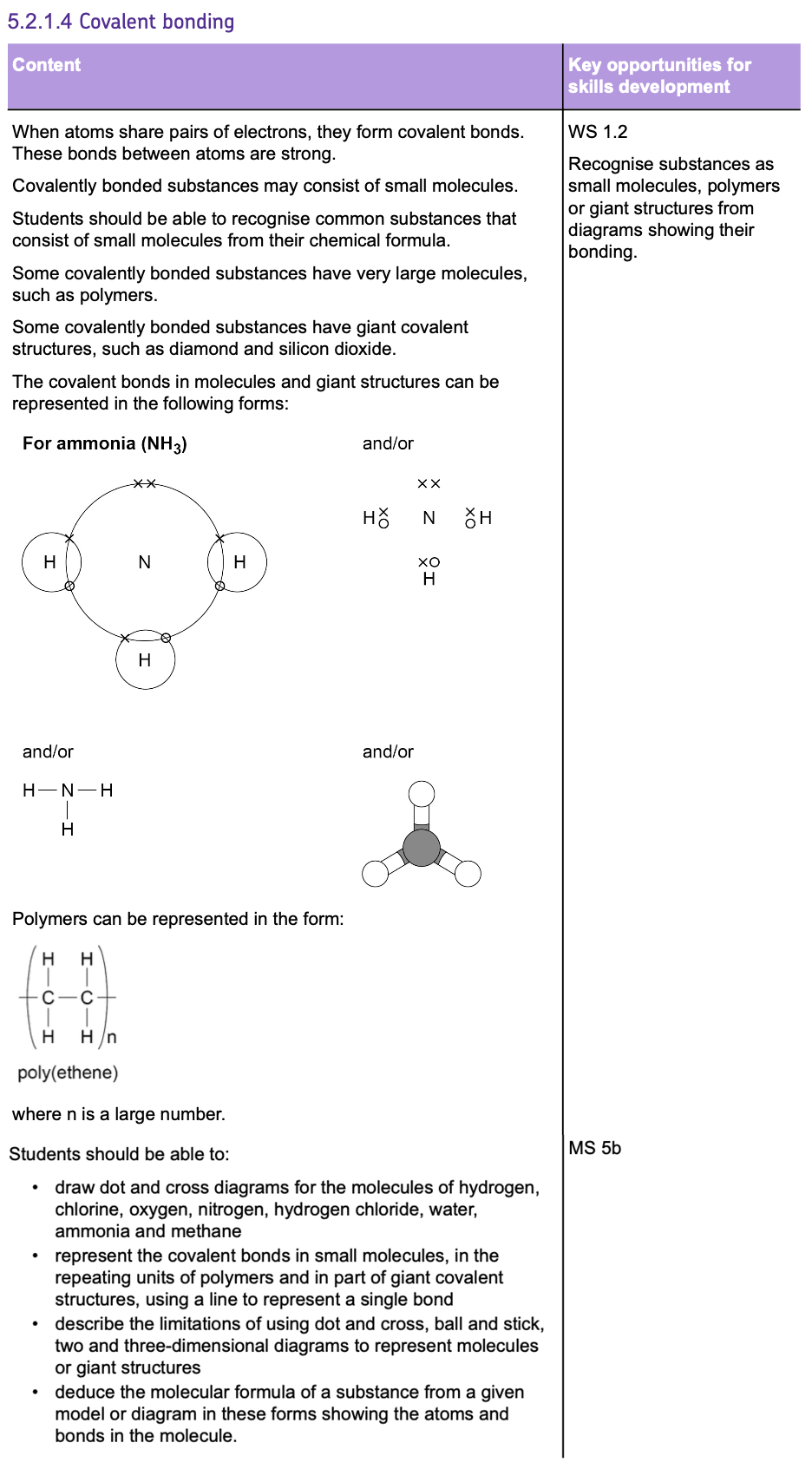
Covalent Bonding
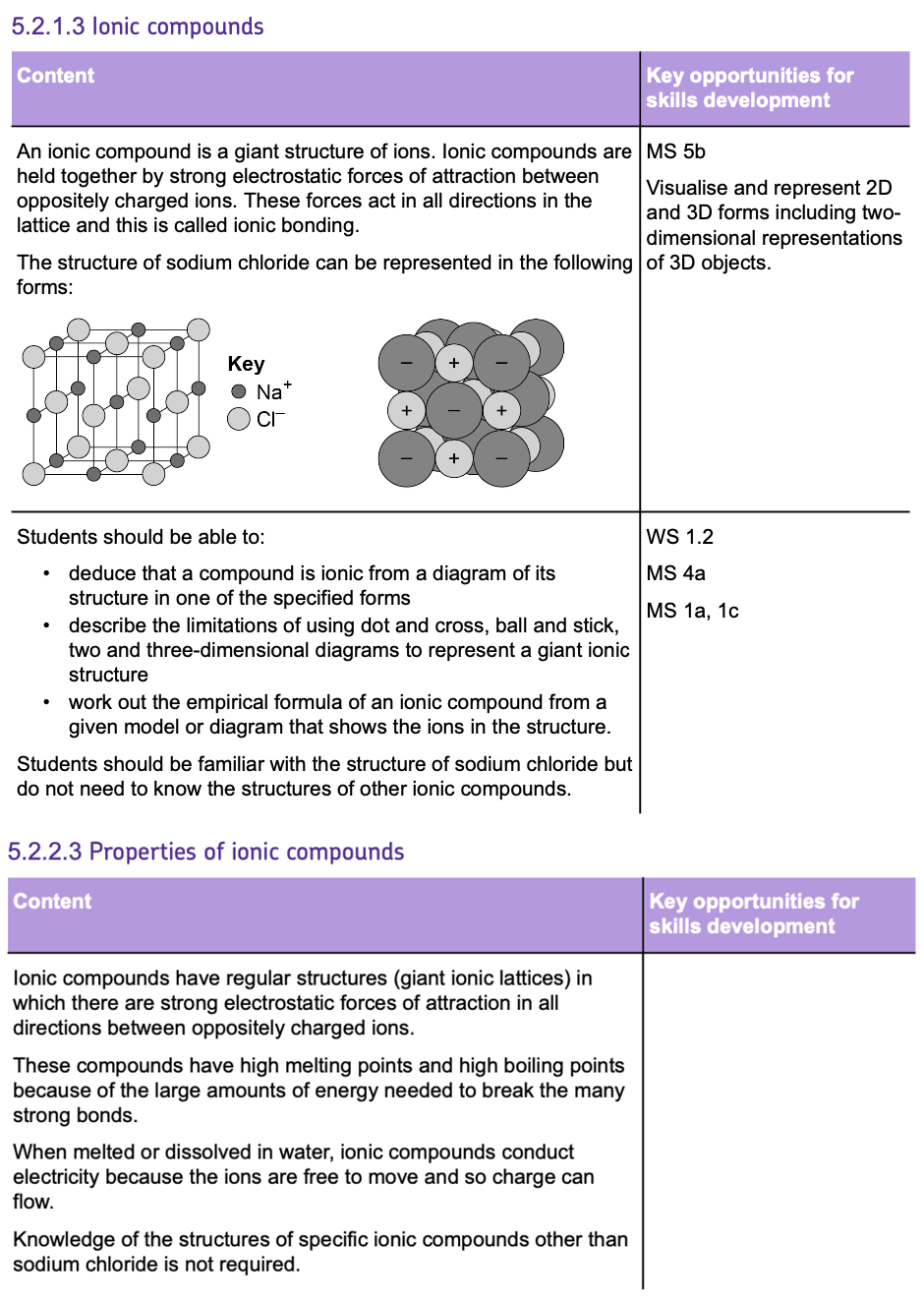
- This type of bonding occurs between NON-METALS.
- COVALENT BONDING involves atoms SHARING pairs of electrons, so that the atoms can achieve a FULL OUTER SHELL and become STABLE.
- These bonds form because the positively charged NUCLEI are attracted to the shared pairs of ELECTRONS by ELECTROSTATIC FORCES.
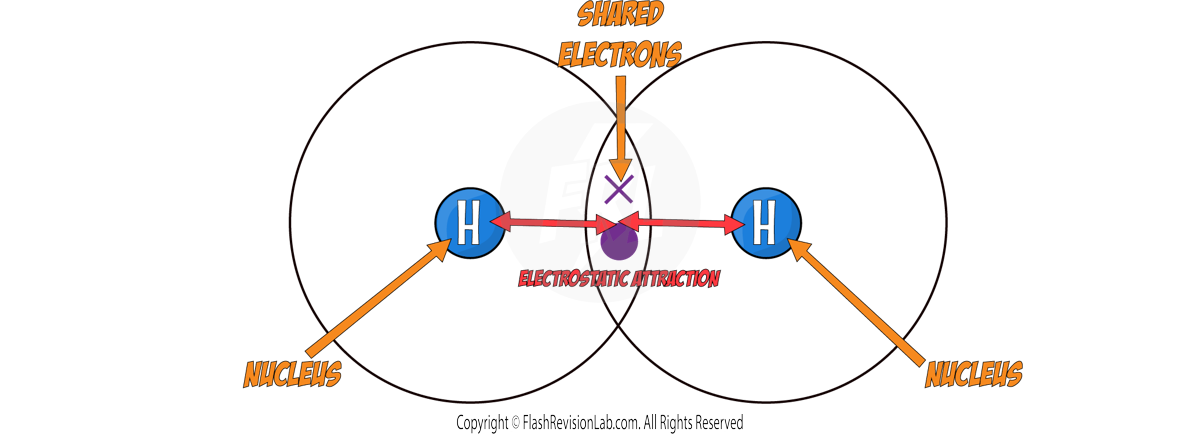
DOT AND CROSS DIAGRAMS
- In these diagrams you only need to show the OUTER SHELL.
- To represent atoms SHARING ELECTRONS, the dot and cross diagrams have OVERLAPPING SHELLS where the shared electrons are drawn.
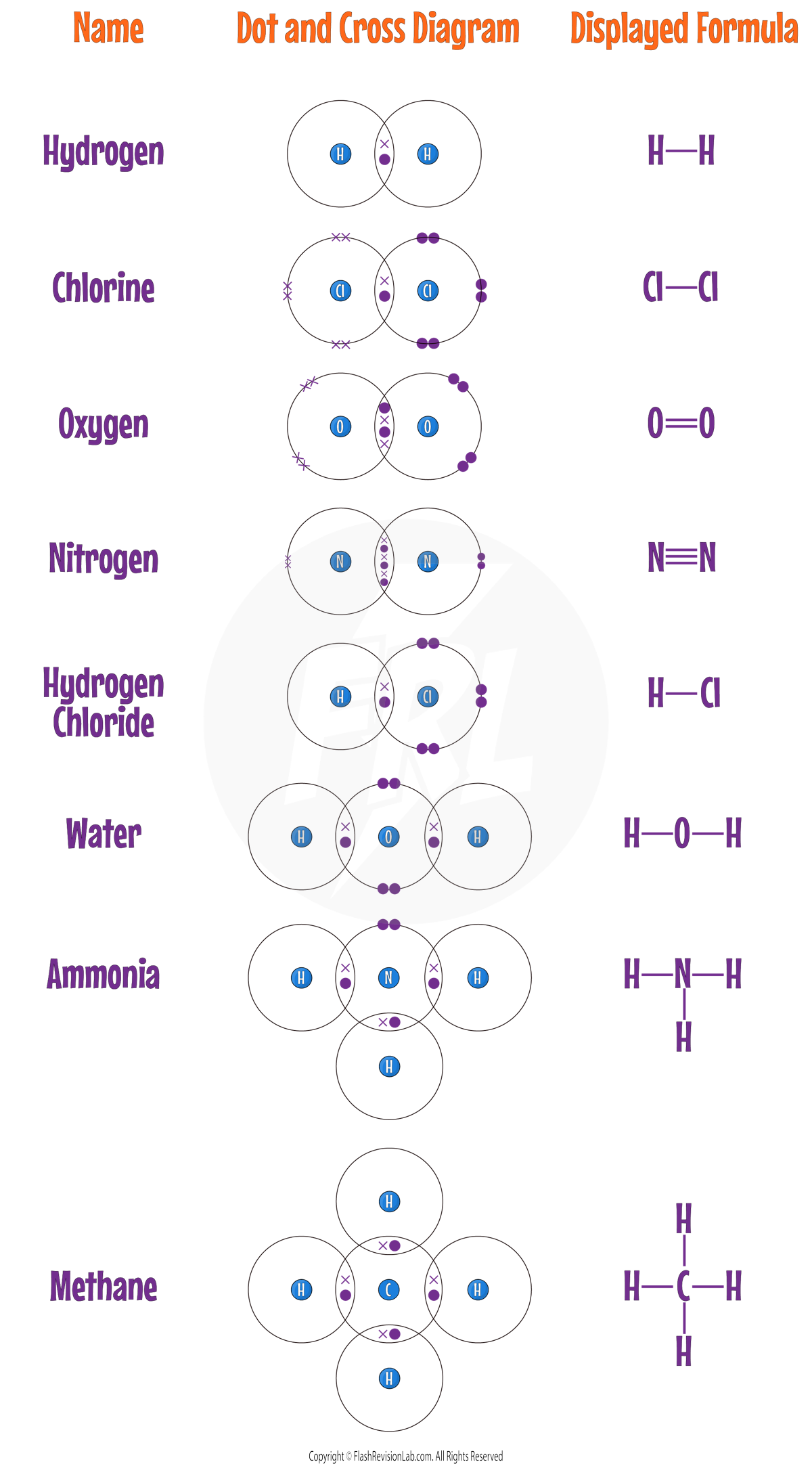
DISPLAYED FORMULA:
These are simplified diagrams that use LINES to represent COVALENT BONDS.

Here are ALL the covalent diagrams you need to know:
3D MODELS:
These can help visualise the spatial arrangement of atoms and bonds in a molecule, offering insight into its structure.
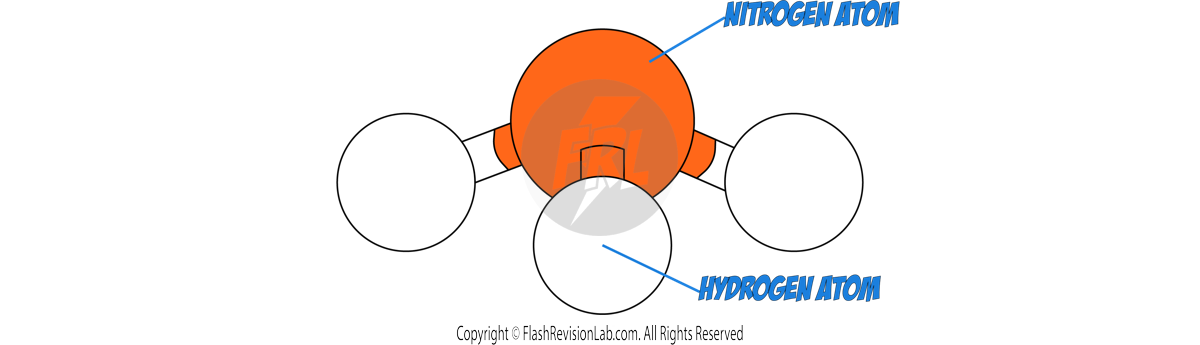
To determine the MOLECULAR FORMULA of a compound, count the number of each type of atom present in the molecule using any representation.
As there are 3 HYDROGEN atoms and 1 NITROGEN atom, this molecule has a formula of NH3.
Here is a comparison of the advantages and disadvantages of using these diagrams to represent covalent substances:
Covalent Compounds
There are THREE types of substance made out of COVALENT bonds:
1. SIMPLE MOLECULAR SUBSTANCES
2. GIANT COVALENT SUBSTANCES
3. POLYMERS
Simple Molecular Substances (Small Molecules)
Simple molecular substances consist of SMALL MOLECULES formed by COVALENT BONDS.
These are NOT large networks but SMALL GROUPS of atoms bonded together.
Familiar examples include, Hydrogen (H₂), Oxygen (O₂), Water (H₂O) and Chlorine (Cl₂).
Properties of Simple Molecular Substances
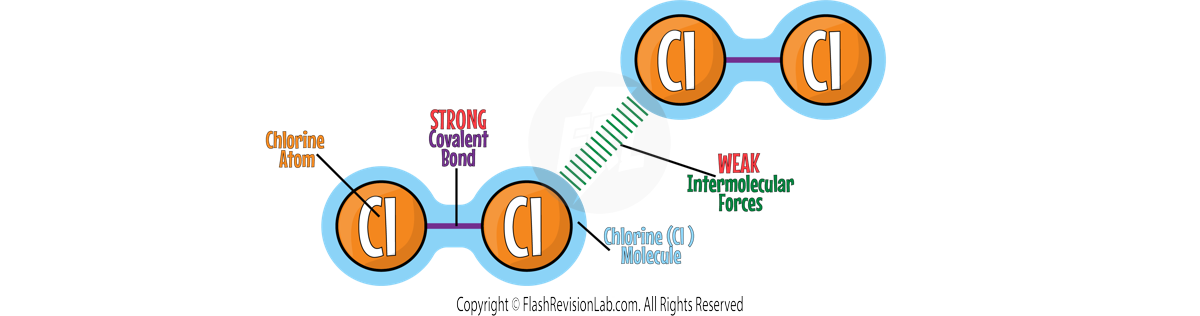
There are TWO bonds involved in simple molecular substances:
1. COVALENT BONDS
These are the bonds BETWEEN ATOMS which are STRONG and need A LOT of energy to overcome.
2. INTERMOLECULAR FORCES:
These are the forces BETWEEN MOLECULES which are WEAK and do NOT need a lot of energy to overcome.
Melting and Boiling Points:
When simple molecular substances MELT or BOIL, it is the INTERMOLECULAR FORCES that need to be broken, NOT the covalent bonds.
These intermolecular forces are WEAK, meaning only a SMALL amount of energy is needed to overcome them.
This gives simple molecular substances LOW MELTING and BOILING points.
This is why they are usually found as LIQUIDS or GASES at room temperature.
Strength of Intermolecular Forces:
Intermolecular forces are always WEAK when compared to COVALENT bonds, but some intermolecular forces are weaker than others.
The LARGER the molecule, the STRONGER the intermolecular forces. This is because the molecule has MORE ELECTRONS.
Let’s compare the boiling points of Chlorine and Iodine:
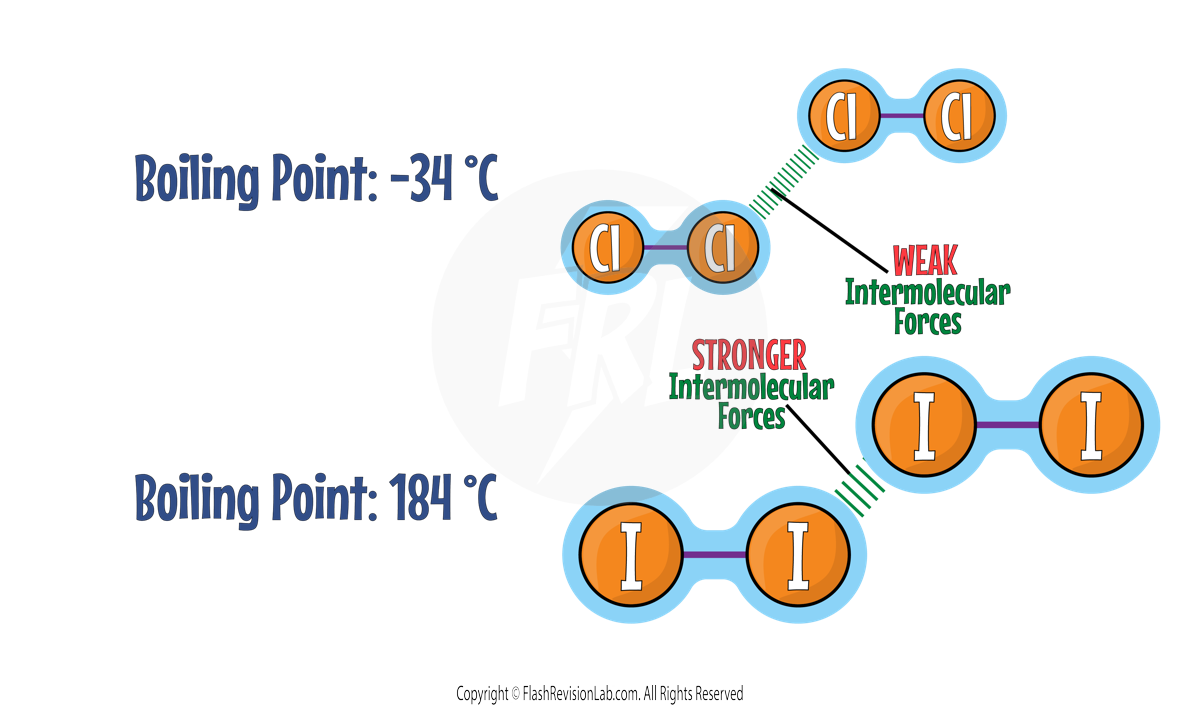
Iodine atoms are LARGER as they are further down the periodic table.
This means Iodine molecules have MORE ELECTRONS, meaning there are STRONGER INTERMOLECULAR forces between the molecules.
MORE ENERGY is required to OVERCOME these forces which means Iodine has a HIGHER melting and boiling point than Chlorine.
Electrical Conductivity:
For a substance to conduct electricity, it needs to contain CHARGED PARTICLES that are free to move (usually electrons or ions).
Simple molecular substances do NOT have an overall electric charge, therefore these substances generally do not conduct electricity because they do not contain FREE MOVING ELECTRONS OR IONS.
Polymers
Polymers are long chains of REPEATING UNITS known as monomers.
These atoms within the chains are held together by COVALENT BONDS, creating LARGE MOLECULES with unique properties.
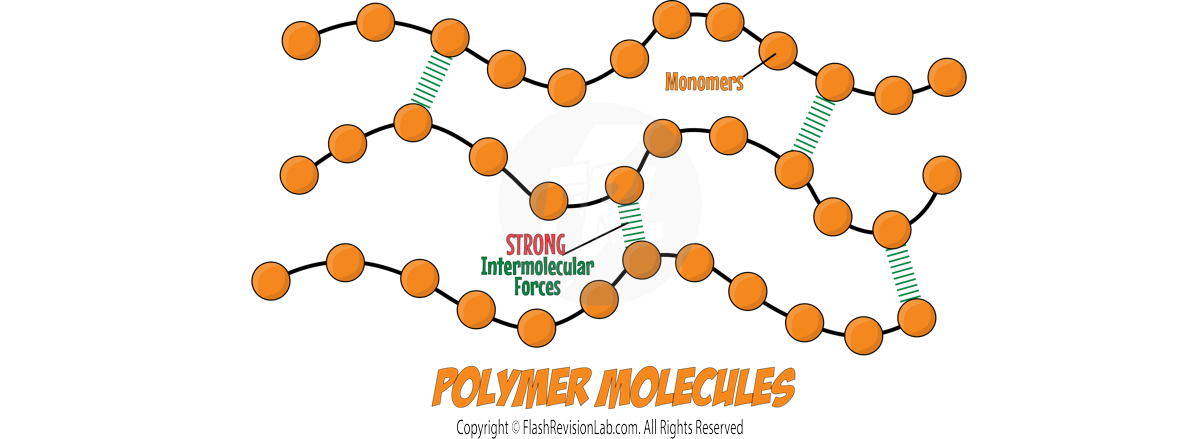
These LONG CHAINED MOLECULES are very LARGE so have very STRONG INTERMOLECULAR FORCES which require A LOT of energy to overcome.
This results in polymers having HIGH melting and boiling points which means they are usually SOLIDS at room temperature.
Polymers can be represented as molecular formulas using the following format:
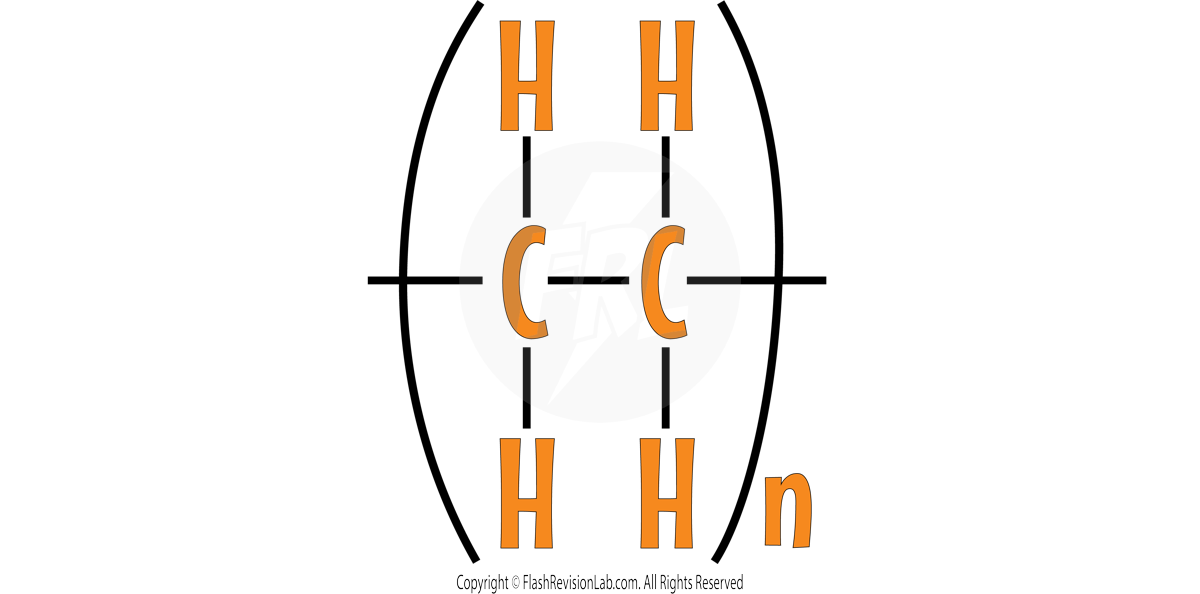
This shows the REPEATING UNIT of the polymer poly(ethene). The structure within the brackets is repeated several times to give the structure of the polymer.
The molecular formula of poly(ethene) is (C₂H₄)n.
Giant Covalent Structures
These are structures where atoms are bonded in a LARGE NETWORK OF COVALENT BONDS.
Every single atom in these substances is bonded to another with STRONG COVALENT BONDS.
![]()
They have HIGH MELTING AND BOILING POINTS because a LARGE AMOUNT OF ENERGY is required to break the strong covalent bonds in the network.
Examples: Diamond, Graphite, and Silicon Dioxide (Silica) are prime examples of giant covalent structures.
Allotropes of Carbon
Allotropes are DIFFERENT STRUCTURAL forms of the SAME ELEMENT in the same physical state.
Here are examples of different allotropes of Carbon:
Diamond
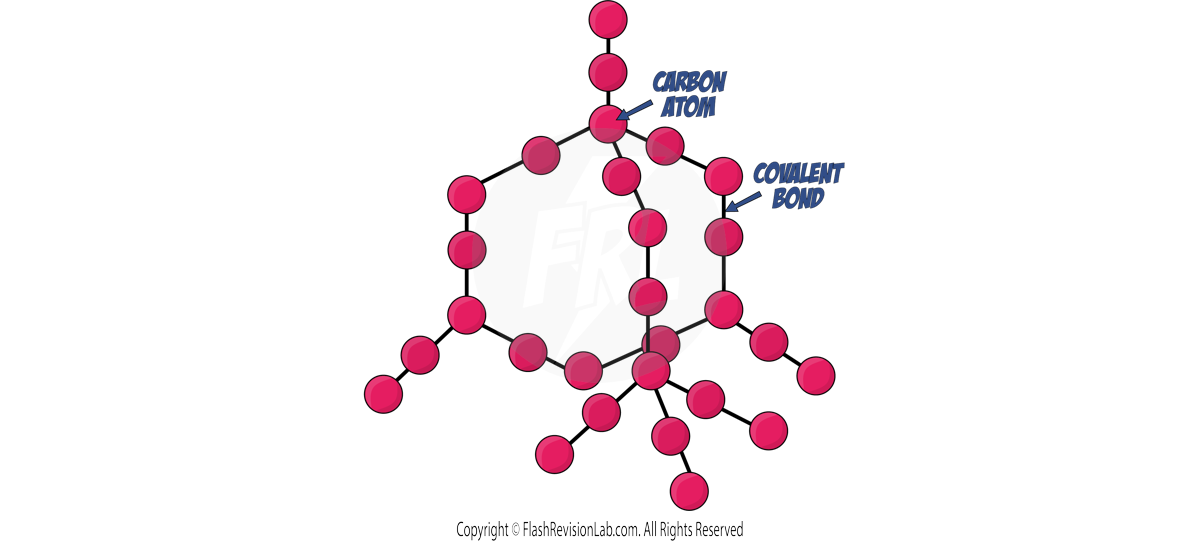
- Formed from CARBON ATOMS each sharing four COVALENT BONDS in a rigid, three-dimensional structure.
- DIAMOND is very hard, with a high melting point. This is because of the LARGE NETWORK of covalent bonds that require a LARGE AMOUNT of ENERGY to overcome.
- It does not conduct electricity as it has NO FREE MOVING ELECTRONS OR IONS.
Graphite
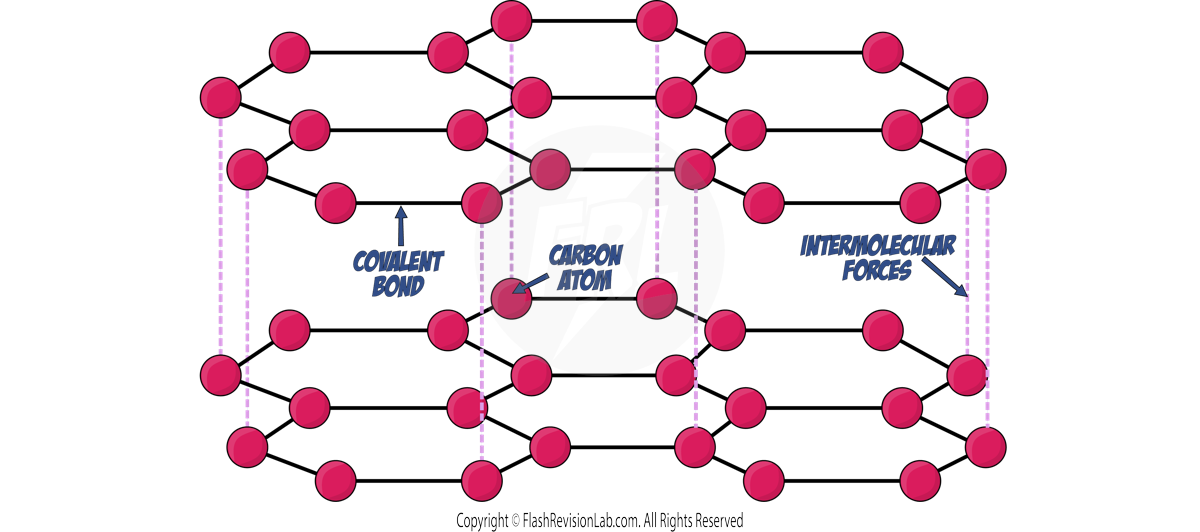
- Composed of LAYERED structures of HEXAGONAL RINGS, with each carbon atom bonded to THREE other atoms with COVALENT BONDS.
- Carbon has FOUR electrons in its outer shell, so the fourth electron that is NOT used for bonding is DELOCALISED.
- This means GRAPHITE is a good CONDUCTOR of electricity, as its delocalised electrons can carry a charge (just like metals).
- It is SOFT, and used as a LUBRICANT due to weak forces between layers, causing the layers to SLIDE over one another.
Graphene
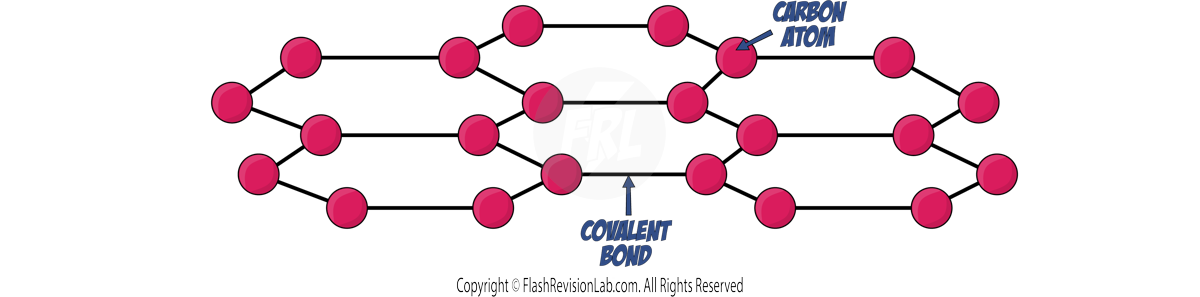
- Graphene ia a SINGLE LAYER of graphite, and is strong and light.
- It is a good CONDUCTOR of HEAT and ELECTRICITY due to its DELOCALISED ELECTRONS, so is used in ELECTRONICS and composite materials.
Fullerenes
- FULLERENES are molecules with hollow shapes, such as spheres or tubes.
- The structure of fullerenes is based on hexagonal rings of Carbon atoms but they may also contain rings with five or seven Carbon atoms.
- They can ENCAPSULATE other molecules and are used in drug delivery and as INDUSTRIAL CATALYSTS.
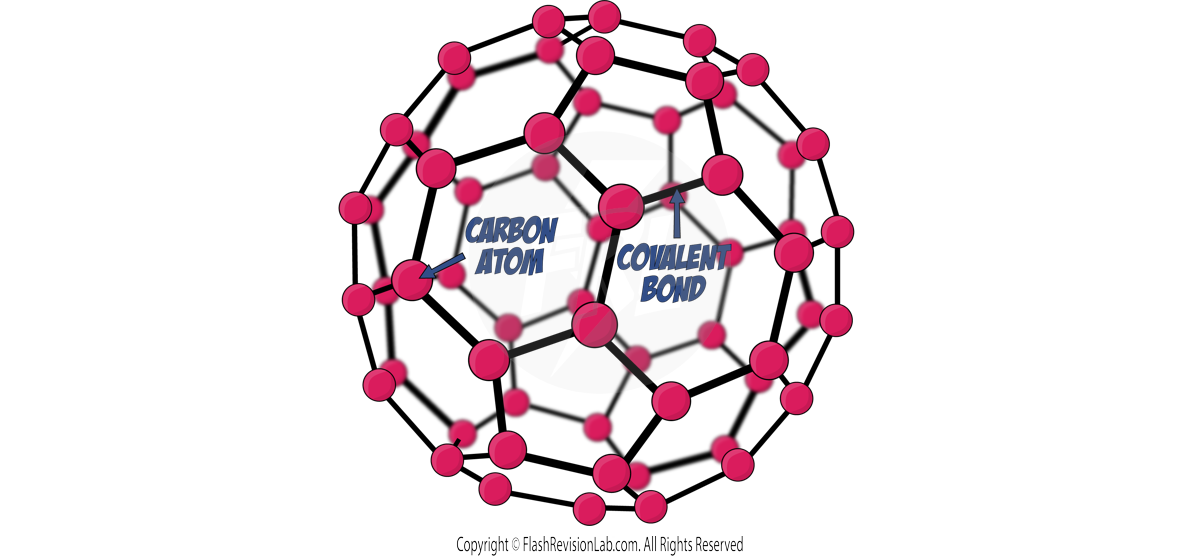
- BUCKMINSTERFULLERENE, is a SPHERICAL FULLERENE with a formula of C60.
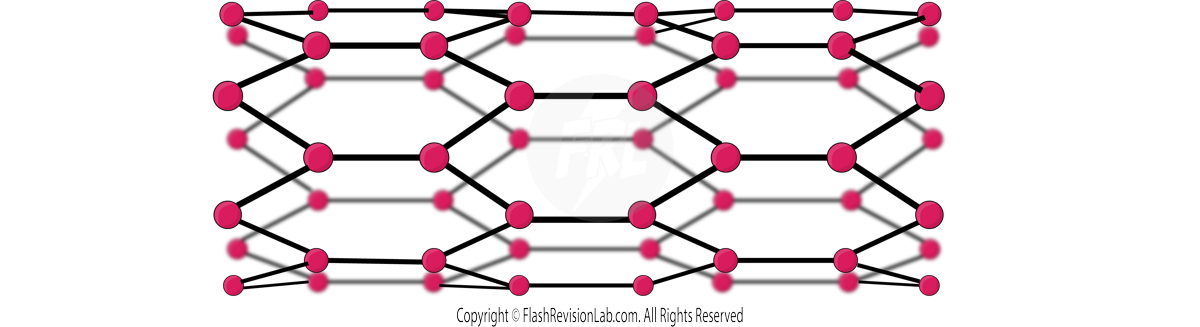
- Carbon nanotubes are CYLINDRICAL fullerenes with very HIGH length to diameter ratios.
- Their properties make them useful for nanotechnology, electronics and materials.
- They are good CONDUCTORS of HEAT and ELECTRICITY as they have DELOCALISED ELECTRONS, meaning they can be used in ELECTRONICS and nanotechnology.
- They have a very high TENSILE STRENGTH without much MASS meaning they are useful for certain materials, such as the ones used in TENNIS RACKETS.
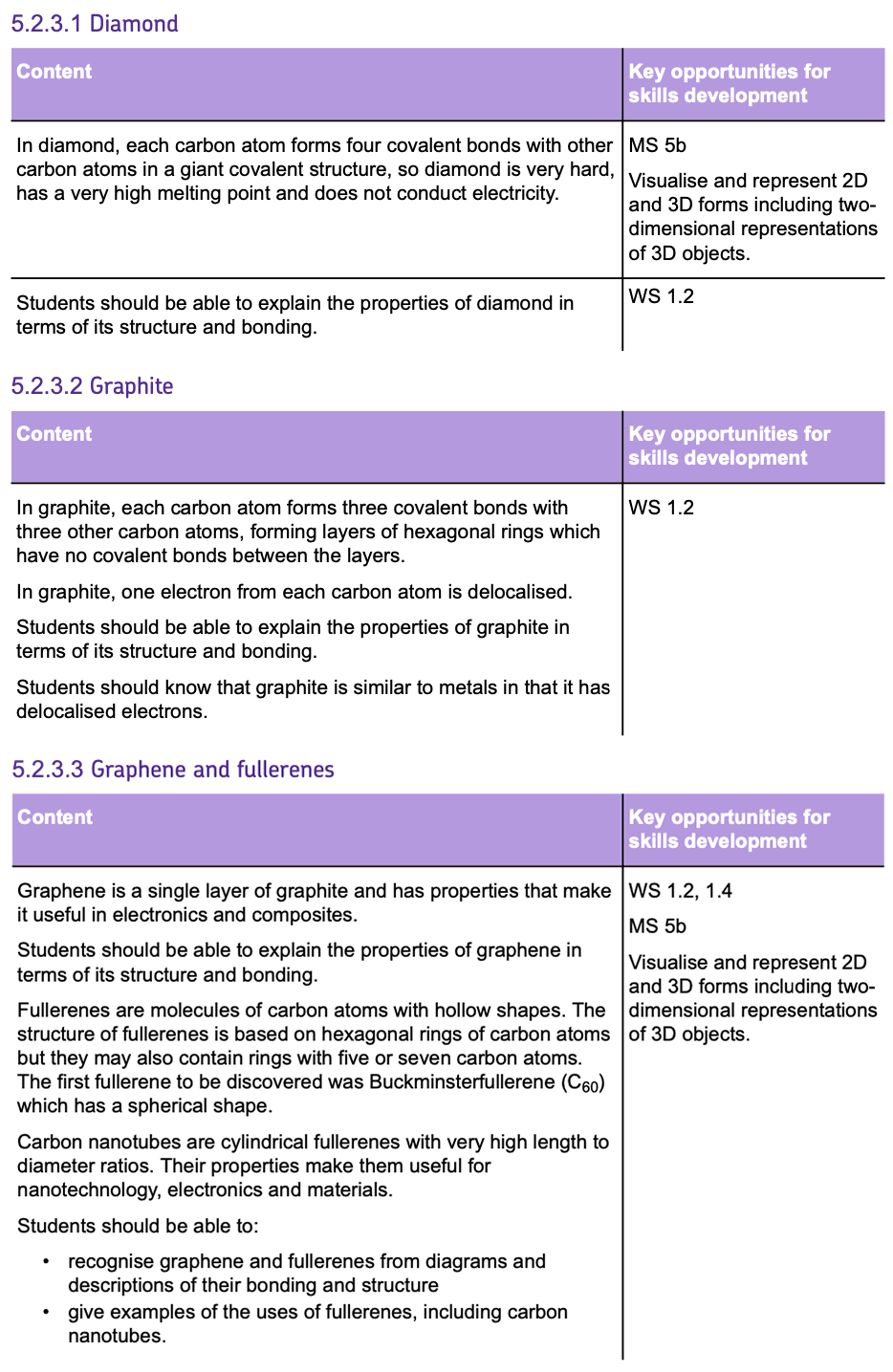
Metallic Bonding
- This type of bonding occurs between METALS.
- The outer shell electrons are DELOCALISED and free to move. This creates a SEA OF DELOCALISED ELECTRONS.
- Within the sea of electrons, there are POSITIVE METAL IONS which are arranged in a REGULAR PATTERN in a GIANT STRUCTURE.
- The ELECTROSTATIC ATTRACTION between the POSITIVE METAL IONS and the SEA OF DELOCALISED ELECTRONS are the METALLIC BONDS which hold the structure together.
PROPERTIES OF METALS
1. HIGH MELTING POINTS
- There are STRONG ELECTROSTATIC FORCES of attraction between positive metal ions and the SEA OF DELOCALISED ELECTRONS.
- This requires a LARGE AMOUNT OF ENERGY to overcome.
- This means their melting points are high and metals are generally SOLID at ROOM TEMPERATURE.
2. GOOD CONDUCTIVITY OF ELECTRICITY
Metals are good conductors of electricity because the DELOCALISED ELECTRONS in the metal CARRY AN ELECTRICAL CHARGE through its structure.
3. GOOD CONDUCTIVITY OF HEAT
Metals are good conductors of THERMAL ENERGY because energy is TRANSFERRED well by the delocalised electrons.
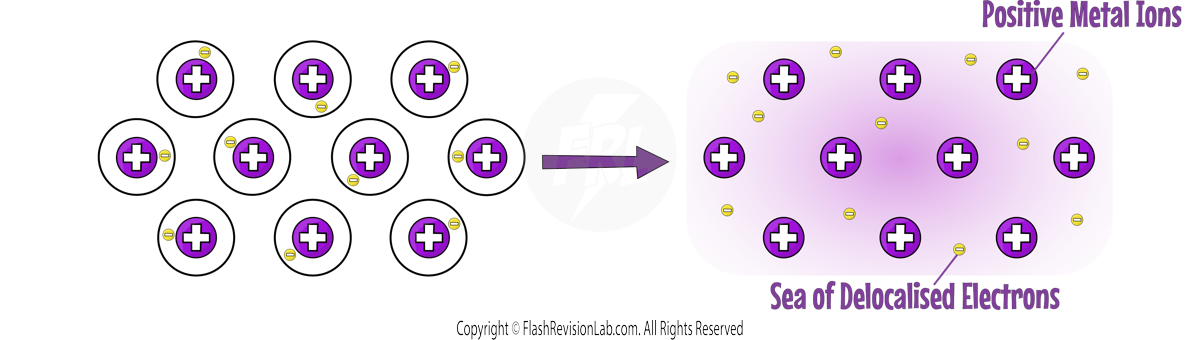
4. MALLEABLE:
This means they can be BENT or SHAPED without breaking, due to the LAYERS of atoms that can SLIDE over each other.
PURE metals are too soft for many uses, so are turned into ALLOYS which are more useful.
ALLOYS are MIXTURES of different metals.
ALLOYS are harder materials than PURE metals because the different sizes of atoms DISTORT the layers of atoms.
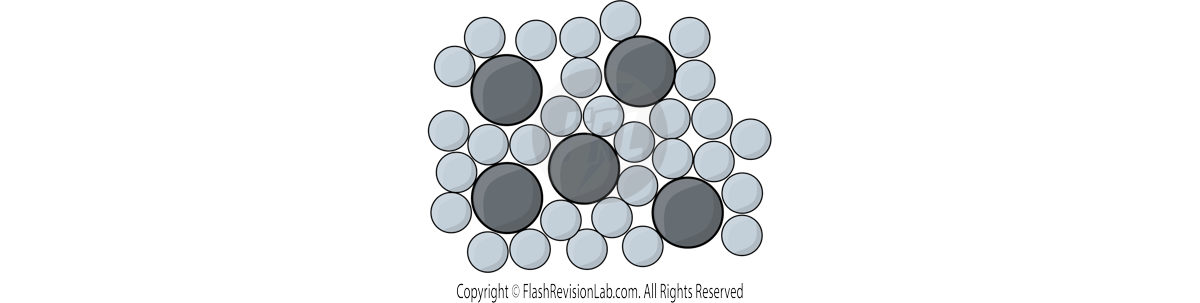
This makes it more difficult for them to SLIDE over each other.
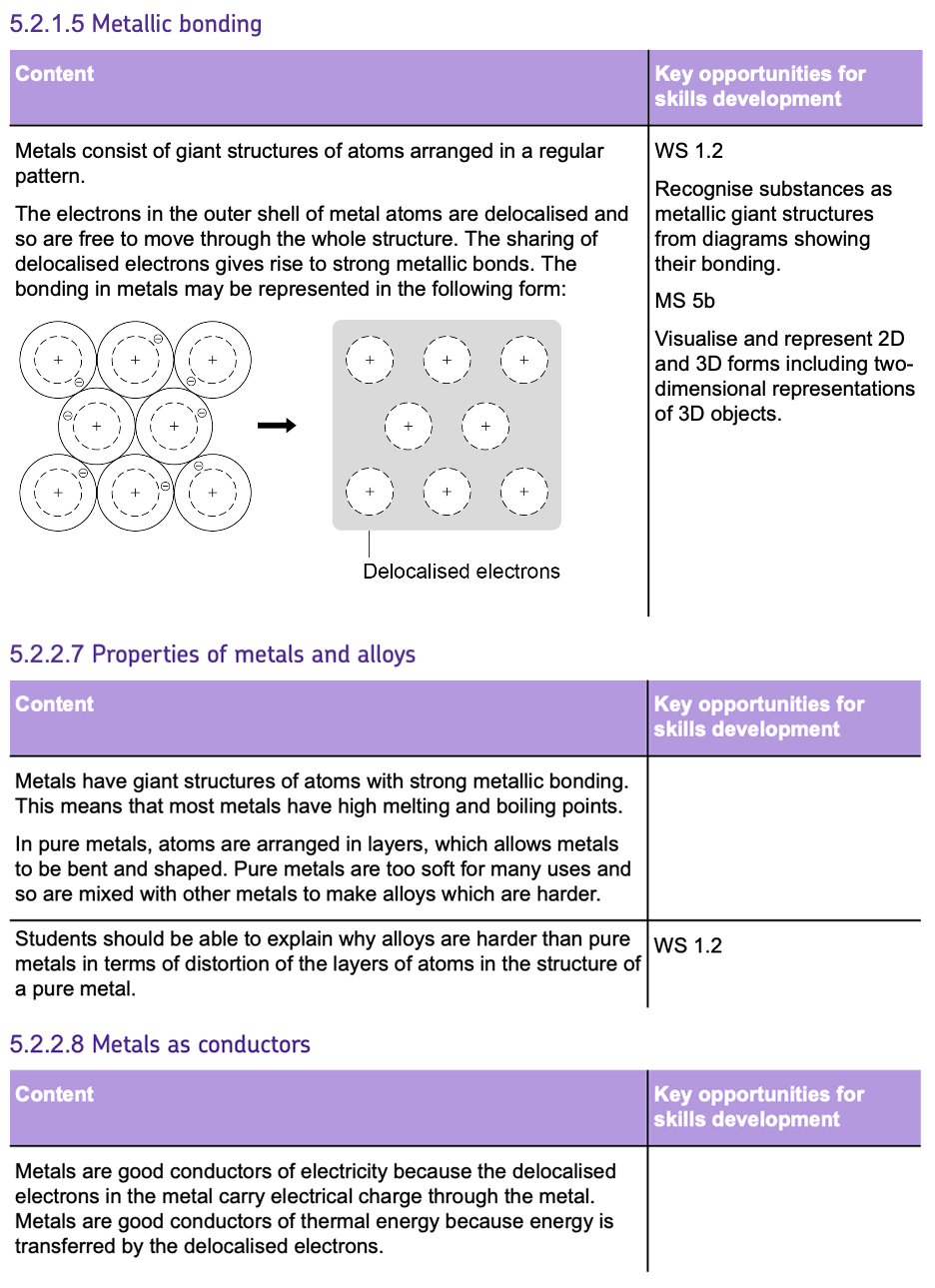
States of Matter
Materials can exist in three states: SOLID, LIQUID, and GAS.
These states can be represented using a SIMPLE MODEL.
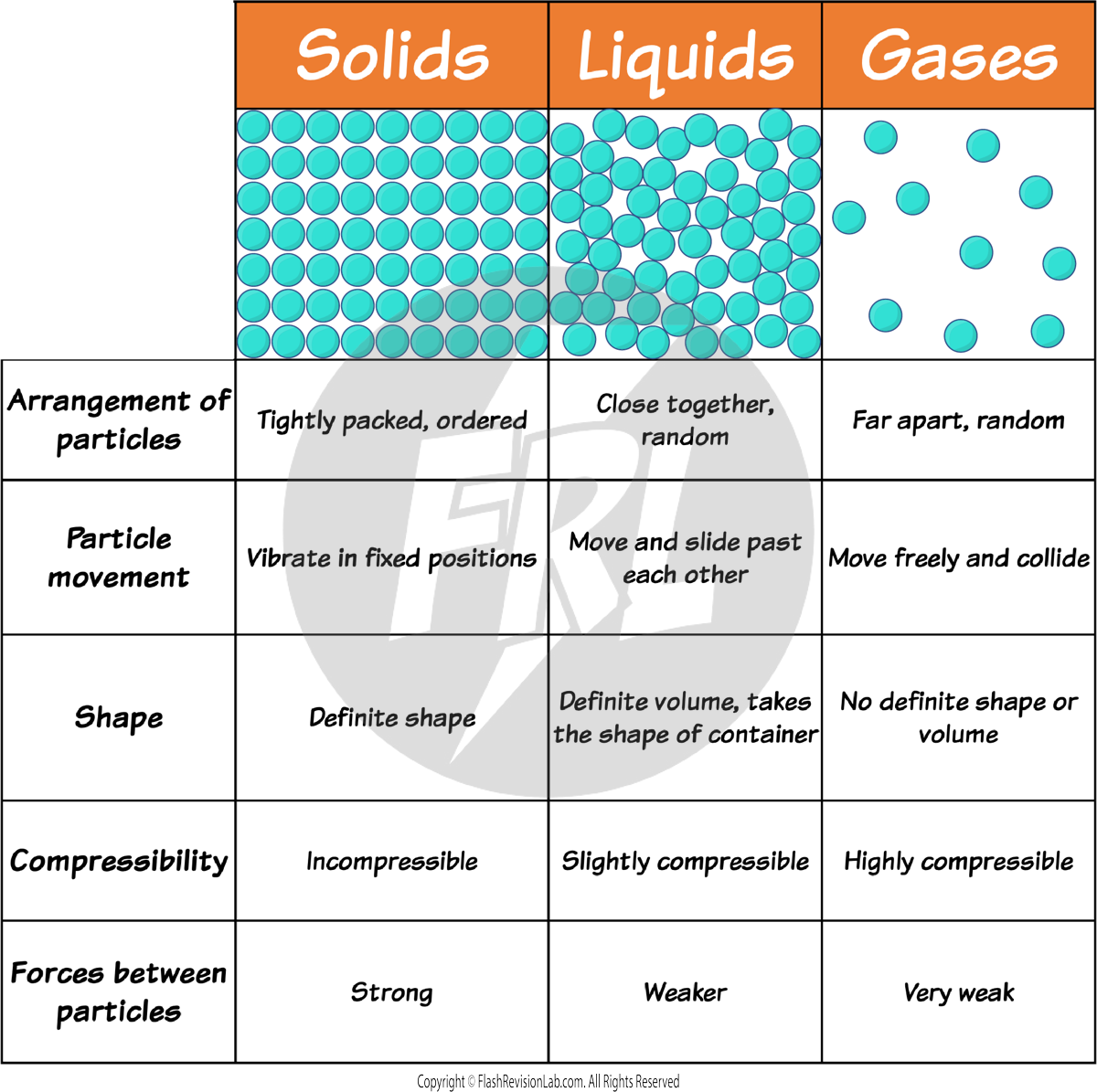
This SIMPLE MODEL explains the behaviour of particles in different states using the model of SMALL, INELASTIC SPHERES.
However, in reality, the particles aren’t SOLID, SPHERICAL or INELASTIC. The particles are atoms, ions or molecules.
The simple model does not show the ACTUAL FORCES between particles, so there is no way of knowing how strong they are.
State Symbols in Equations
State symbols are used in chemical equations to indicate the state of substances:
- (s) for solid
- (l) for liquid
- (g) for gas
- (aq) for aqueous (dissolved in water)
Changing State
CHANGING STATE refers to the process where substances CHANGE between solid, liquid, and gas phases.
This process involves energy changes and particle movement. The amount of ENERGY needed to change state from solid to liquid and from liquid to gas depends on the STRENGTH of the forces between the particles of the substance.
Individual atoms themselves do not share the same properties as bulk matter.
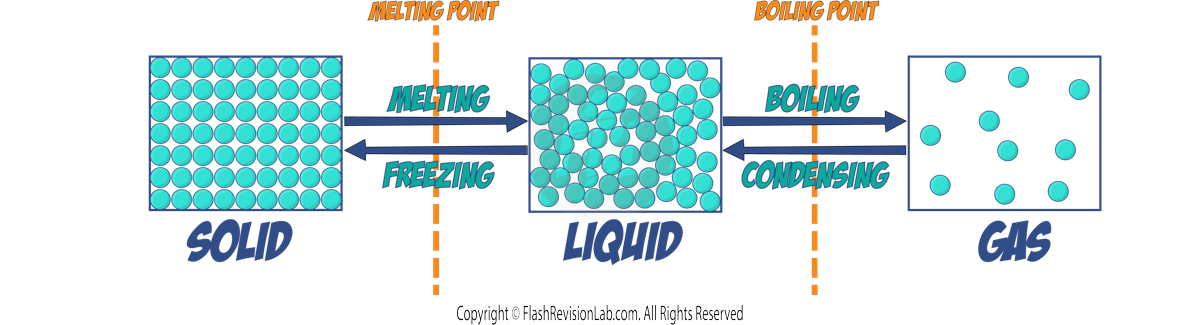
- HEATING A SOLID: When a solid is heated to its MELTING POINT, its particles gain energy and VIBRATE more, weakening the forces holding them together. This is known as MELTING.
- HEATING A LIQUID: Heating a liquid to its BOILING POINT gives particles more energy to move faster, leading to BOILING or EVAPORATING where the liquid becomes a gas.
- FREEZING: When the particles in a liquid are cooled to their MELTING POINT, they lose energy and move less, they form more bonds and the liquid becomes a solid. This is FREEZING.
- CONDENSING: A gas turns into a liquid when it is cooled to its BOILING POINT, as particles lose energy and form bonds.
Particle Theory:
Particle theory explains how matter changes state depending on the ENERGY and FORCES present between the particles in the substance.
- The amount of energy needed to change from a solid to a liquid and from a liquid to a gas depends on the STRENGTH of the FORCES between the particles.
- There are many different types of substances which contain different amounts of elements and compounds.
- Since each substance contains different particles, the amount of energy needed to change the state of them is DIFFERENT for each individual substance.
- The STRONGER the FORCES between the particles, the HIGHER the ENERGY needed for melting and boiling to occur.
- When substances are heated, the particles ABSORB heat energy which causes its particles to VIBRATE more.
- Eventually, when the substance reaches the MELTING POINT, the bonds between the particles break and the solid MELTS into a liquid.
- When it is heated further and the boiling point is reached, the particles gain enough energy for the forces between them to break and they turn into a GAS.
This theory is useful for understanding how changes of state work, but just like the simple model, it has its limitations:
- It assumes all particles to be SOLID, SPHERICAL and INELASTIC, when they aren’t.
- It doesn’t consider the differences caused by different particles, such as atoms, ions and molecules.
- It doesn't consider the INTERMOLECULAR forces between particles in different substances.
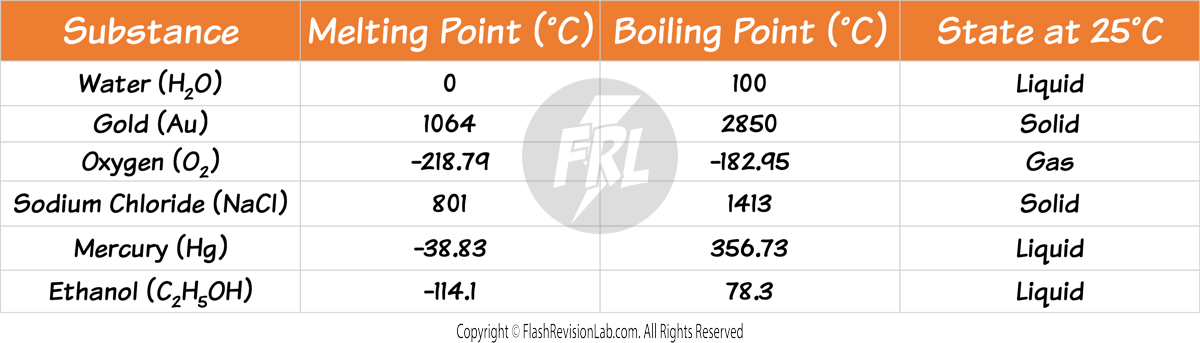
Predicting the State of a Substance
To predict the state of a substance:
- BELOW the MELTING POINT, a substance is SOLID.
- ABOVE the BOILING POINT, it's a GAS.
- BETWEEN these two points, it's a LIQUID.
Let’s predict the states of the following substances at room temperature (25 °C):
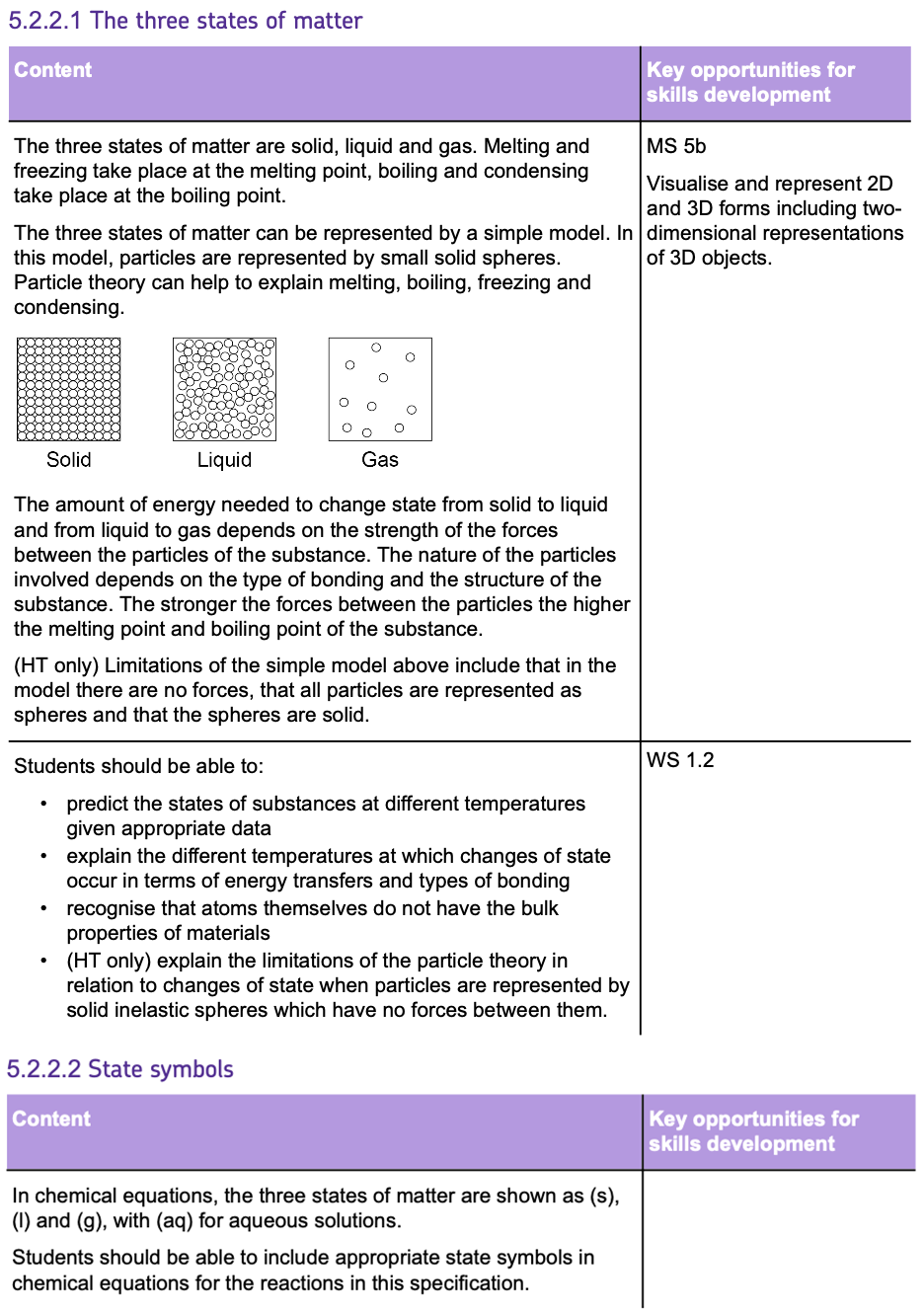
Relative Formula Mass and Percentage Composition
RELATIVE FORMULA MASS
RELATIVE FORMULA MASS (Mr) is the SUM of the RELATIVE ATOMIC MASSES (Ar) of all the atoms in a chemical formula. Here's how to calculate it:
- Find the RELATIVE ATOMIC MASSES (Ar) of each element in the compound (usually listed in the periodic table).
- Multiply the Ar of each element by the NUMBER OF ATOMS of that element in the formula.
- Add these values together to get the Mr of the compound.
For example:
The Mr of CaF2 is calculated as:
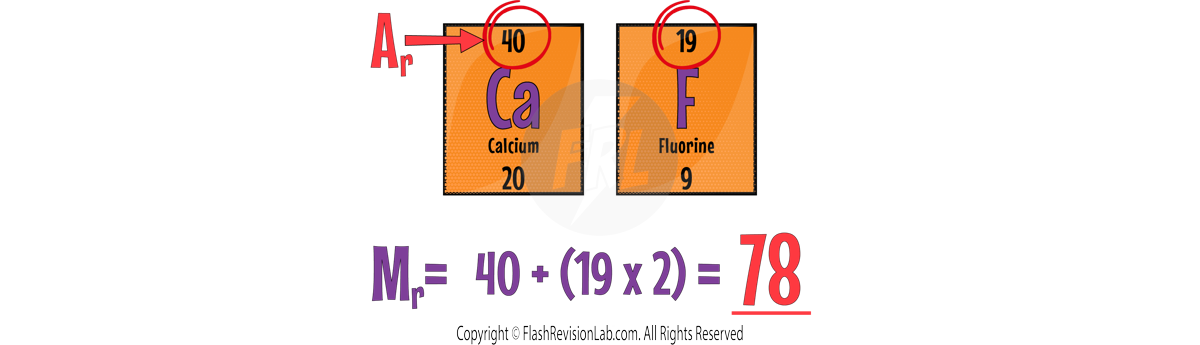
Here are a few more examples:
In a balanced chemical equation, the sum of the relative formula masses of the reactants equals the sum of the relative formula masses of the products.
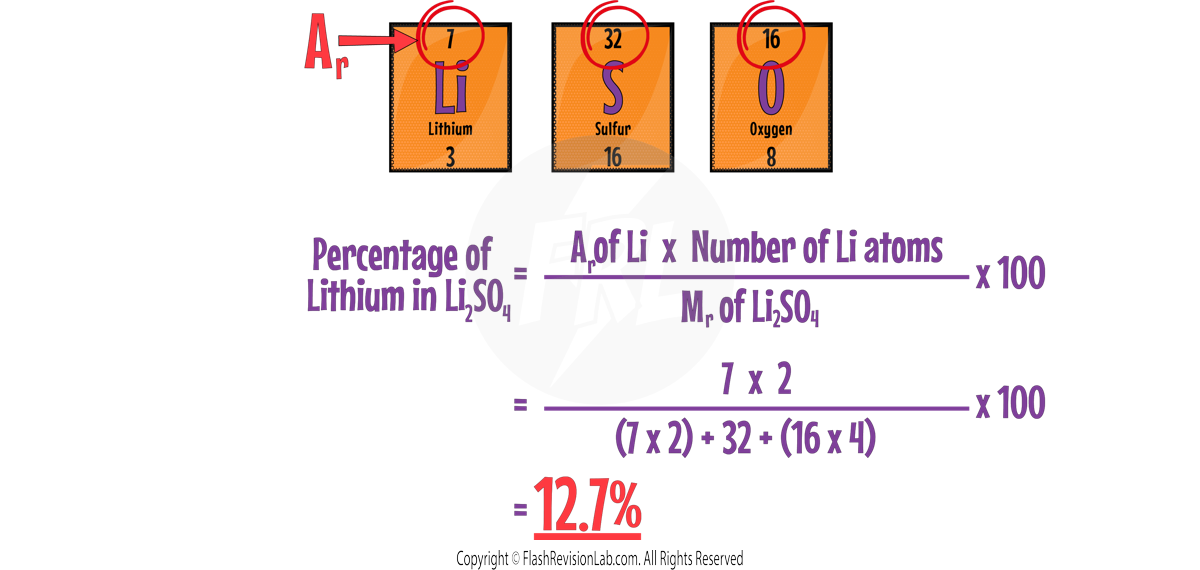
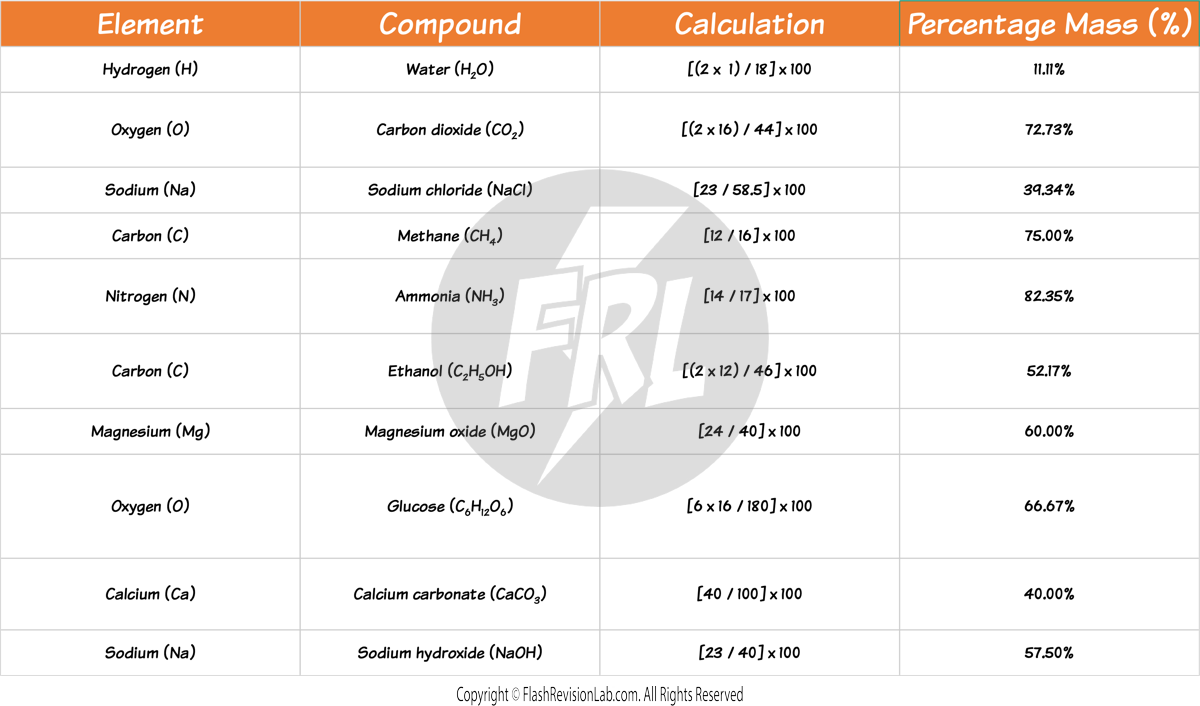
CALCULATING THE PERCENTAGE MASS OF AN ELEMENT IN A COMPOUND
To find the percentage mass of an element in a compound:
- MULTIPLY the RELATIVE ATOMIC MASSES (Ar) of the element by the number of atoms of that element in the compound.
- DIVIDE this by the RELATIVE FORMULA MASS (Mr) of the whole compound.
- Multiply by 100 to convert to a percentage.
For example: The percentage mass of LITHIUM in Li2SO4:
Here are some more examples of finding the percentage of certain elements in compounds:
Conservation of Mass
The Law of Conservation of Mass
In Chemistry, one of the most fundamental principles you'll encounter is the CONSERVATION OF MASS. This means:
- No atoms are CREATED or DESTROYED in a chemical reaction, which means the mass of the reactants EQUALS the mass of the products.
- A balanced chemical equation obeys this law, showing the same number and types of atoms on both sides.
Examples:
- For the reaction:
2Ca + O2 → 2CaO
There are 2 calcium atoms and 2 oxygen atoms BEFORE and AFTER the reaction, demonstrating that mass is conserved.
When Mass Seems to Change
Sometimes, it SEEMS like mass changes during a reaction, but there's always a reason:
1. If mass INCREASES, it could be due to a gas from the air becoming part of the product in an unsealed container.
e.g. when Sodium reacts with Oxygen:

The mass of the Sodium appears to increase. This is because the Oxygen in the air COMBINES with the Sodium to form Sodium Oxide.
The Oxygen’s mass can not be measured as it is a gas, but after it combines, it is a part of the solid so it CAN be weighed.
2. If mass DECREASES, a gas may have formed and ESCAPED from the reaction mixture in a non-enclosed system.
e.g. when Calcium Carbonate is heated in a THERMAL DECOMPOSITION reaction:

It releases Carbon Dioxide GAS which escapes, meaning it can’t be weighed. So when you weigh the products, the mass appears to decrease as the mass of the Carbon Dioxide is not measured once it escapes.
Chemical Measurements
Error
In experiments, an ERROR is the difference between your result and the EXPECTED value.
We have two main error types: RANDOM ERRORS and SYSTEMATIC ERRORS.
Random Errors
This is when measured values vary randomly around the true value. Random error is very common in experiments due to HUMAN ERROR and RANDOM VARIATIONS. By taking many measurements and calculating the MEAN, we can overcome the issue of random errors.
Here's what might cause them:
- Trouble reading the scale of an instrument just right.
- The person taking the reading makes a mistake.
- The environment changes, like the lab getting warmer or the air moving around.
Systematic Errors
This is when an issue in experimental design or EQUIPMENT causes the measured value to be consistently too high or consistently too low. These errors are harder to identify.
Some examples include:
- Forgetting to reset a scale to zero, leading to all your weights being off.
- Not reading a measurement at the correct eye level, so it seems smaller or larger.
Uncertainty
Whenever a measurement is made there is always some uncertainty about the result obtained.
Uncertainty is all about how confident you are in your measurements. It's a number that tells you how much your results might be off by.

Analogue Instruments
With ANALOGUE INSTRUMENTS, uncertainty is usually HALF of the smallest thing you can measure on it.
E.g. If your ruler's smallest division is 1 mm, your uncertainty is ±0.5 mm.
Digital Instruments
For DIGITAL INSTRUMENTS (like a digital clock), it's simpler: uncertainty is just the SMALLEST NUMBER it can display.
Uncertainty in results
- For results that are obtained from a series of REPEATED EXPERIMENTS, the uncertainty is ± HALF of the range of the results.
- This can be estimated by:
- Calculating the MEAN average and then determining the deviation of the highest and lowest results from the mean value.
- An alternative method is to calculate the range of the results and then DIVIDE this value by 2.
Moles
THE MOLE
The MOLE is a measurement for the AMOUNT OF SUBSTANCE of a chemical.
The units for moles is simply: mol.
Definition:
ONE MOLE of any substance has the same number of PARTICLES as AVOGADRO’S CONSTANT.

The value for AVOGADRO’S CONSTANT is 6.02 x 1023 per mole.
The PARTICLES mentioned in this definition can be ATOMS, MOLECULES, IONS and even ELECTRONS.
ONE MOLE of any substance has the SAME MASS in grams as its own RELATIVE FORMULA MASS (Mr).
For Example:
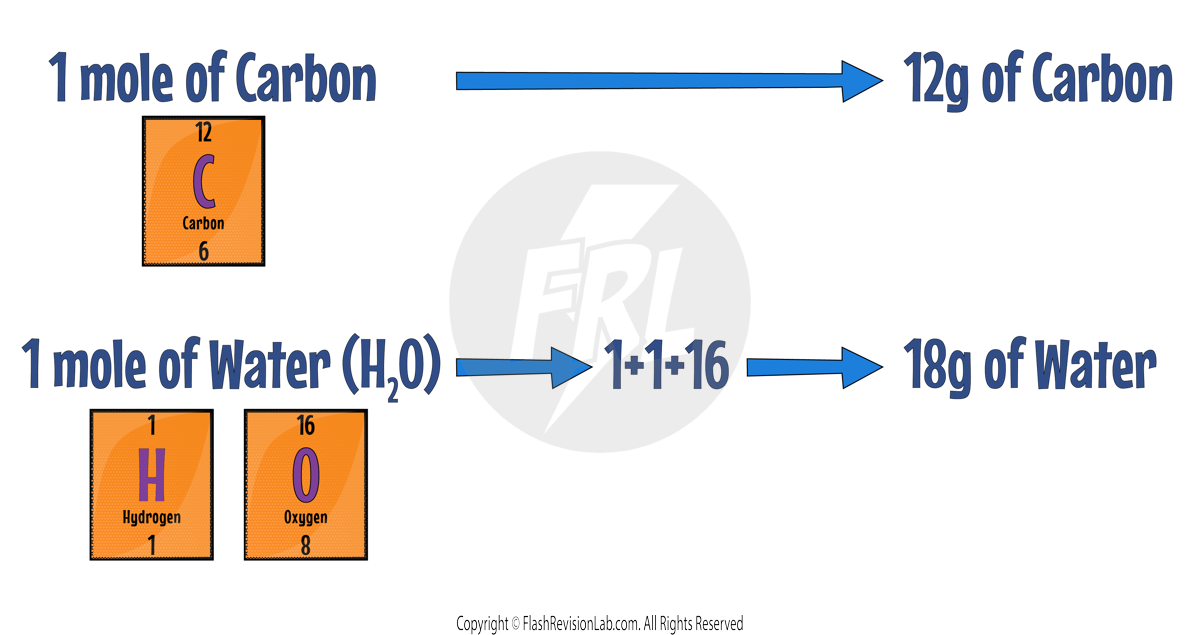
Formula:
The number of moles can be calculated using the formula:

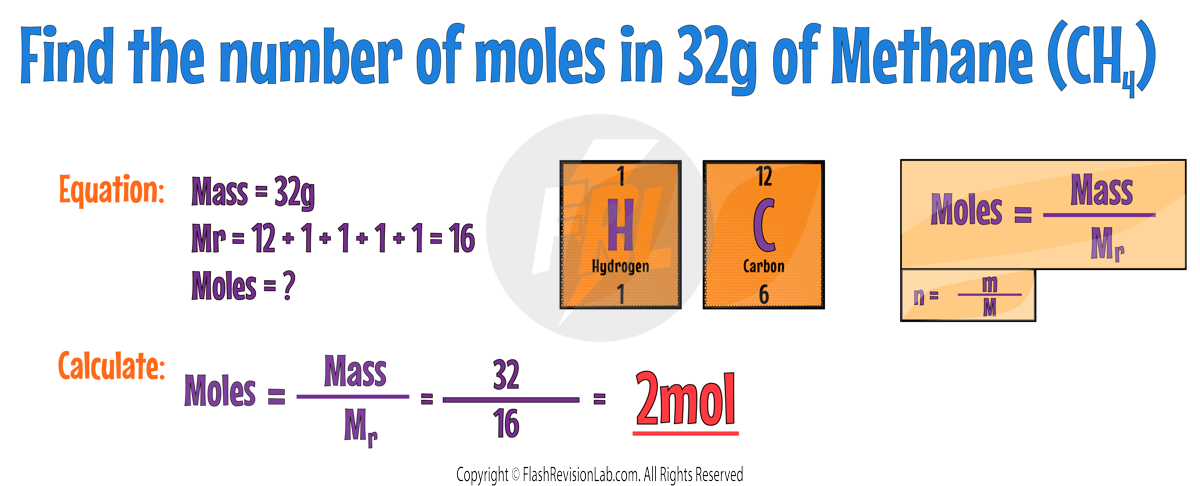
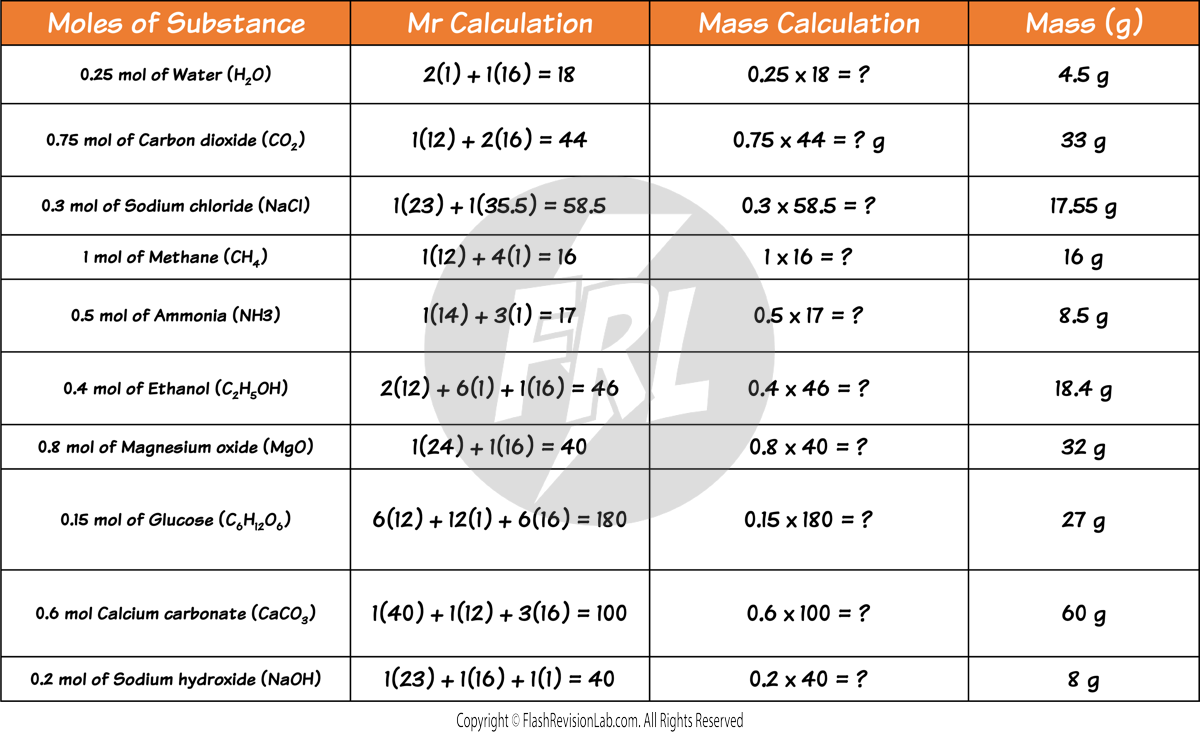
CALCULATING MOLES FROM MASS
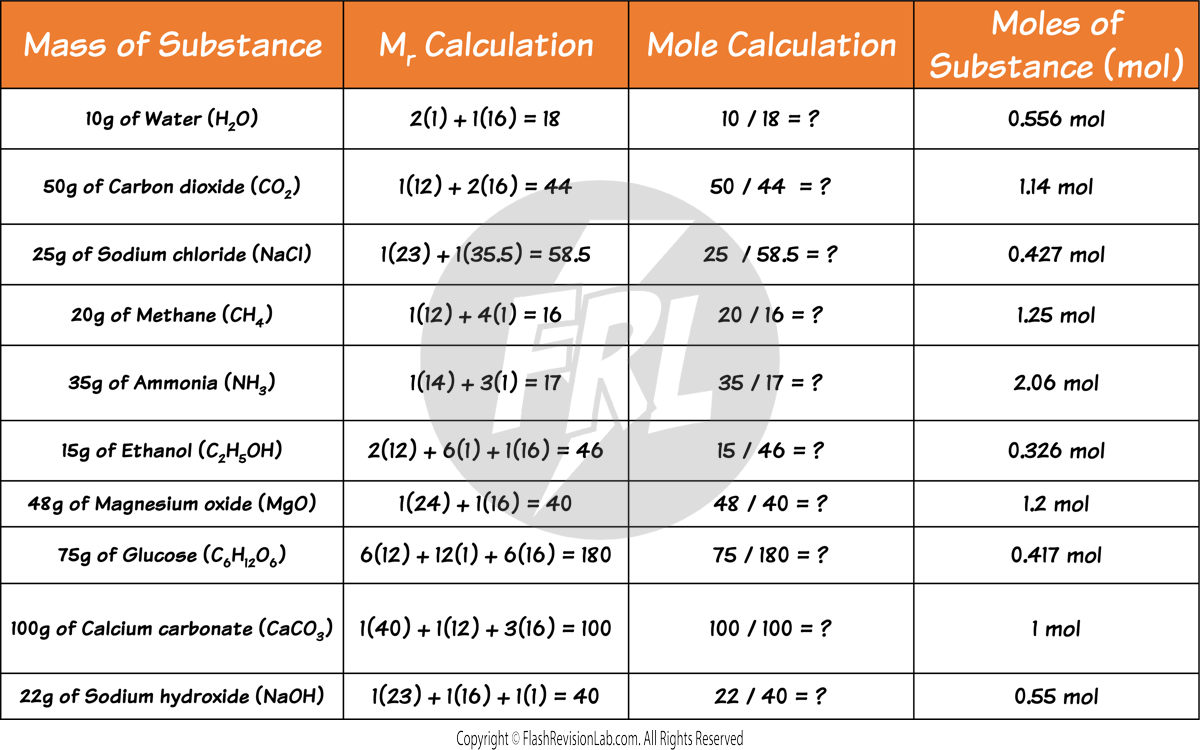
To find the number of moles of a compound from its mass, simply DIVIDE the mass by the compound’s Mr.
Here are some more examples to try:
CALCULATING MASS FROM MOLES
To calculate the mass of a substance from its moles, you can rearrange the formula. This means you can MULTIPLY the moles by the Mr.
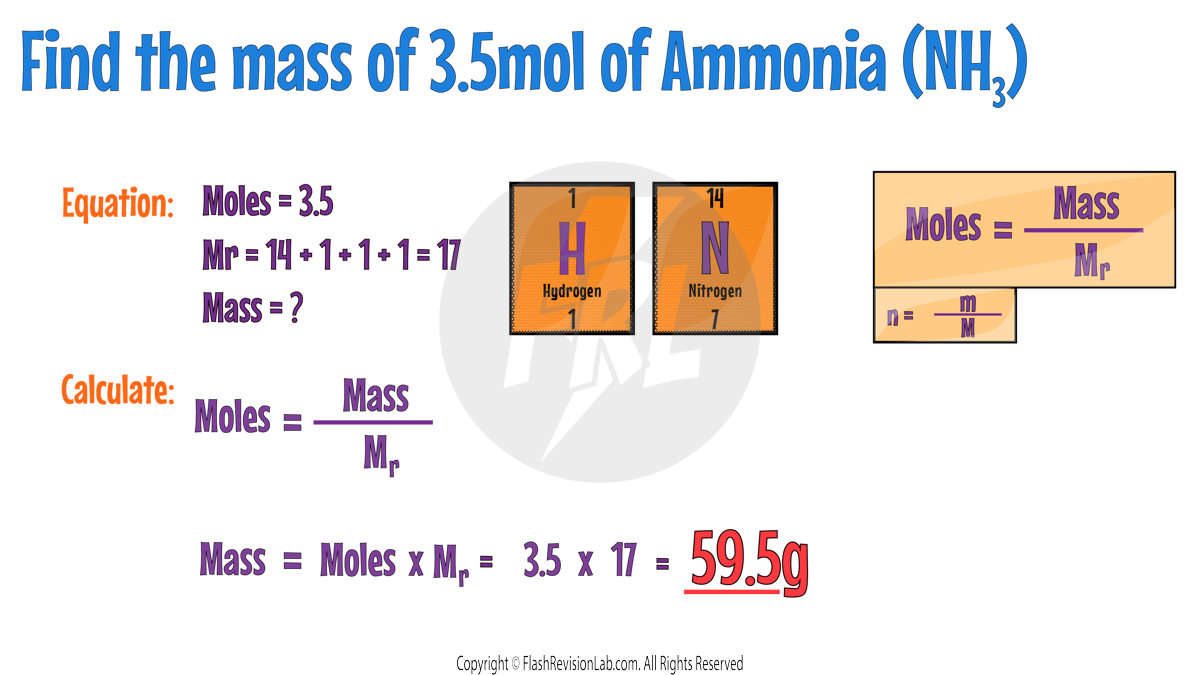
Here are some more examples to try:
The Mole and Chemical Equations
Chemical equations can be used to calculate masses of REACTANTS and PRODUCTS.
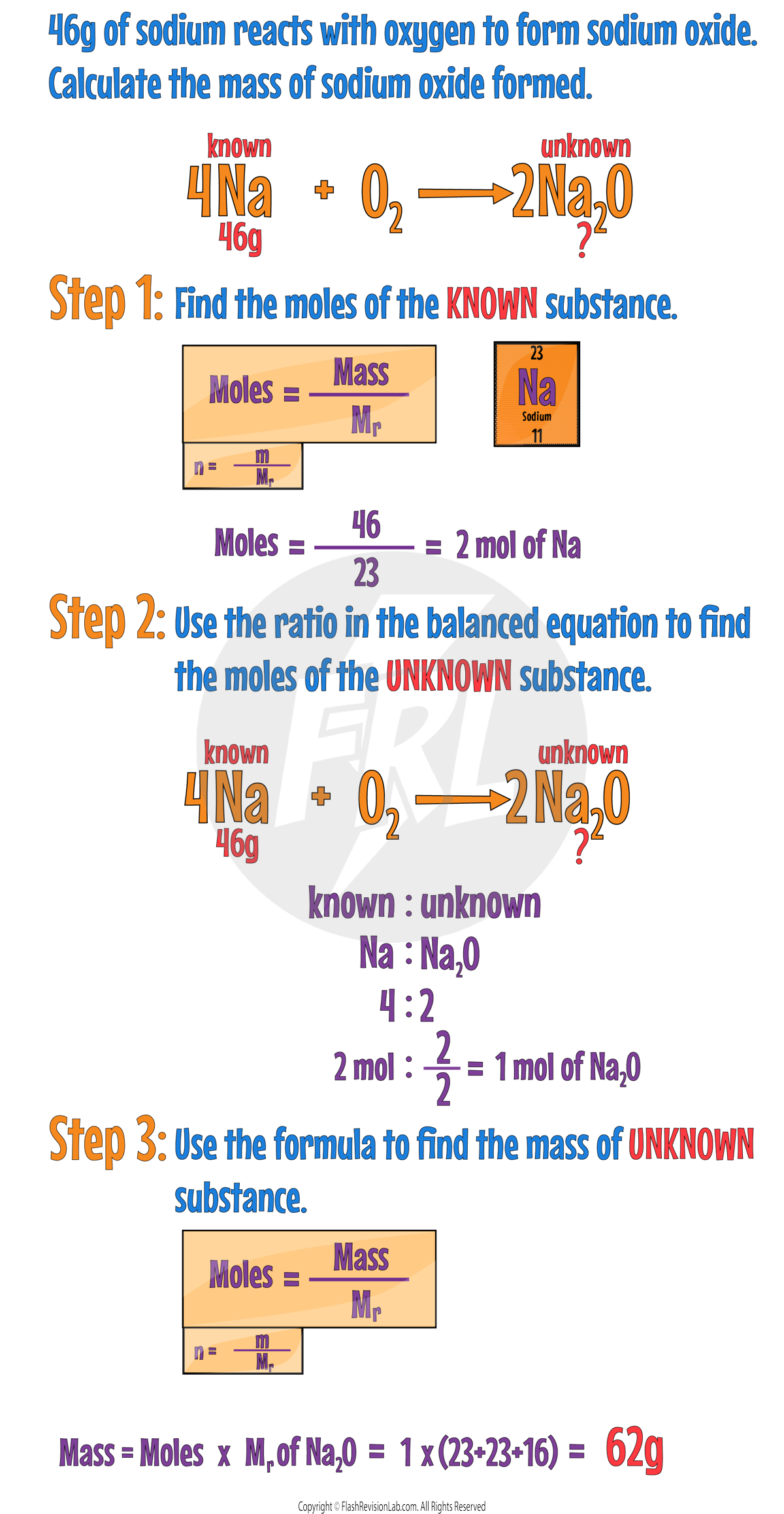
There are a few steps to follow to carry out these calculations.
First identify the KNOWN and UNKNOWN substances.
The KNOWN substance is the substance you know the mass of.
The UNKNOWN substance is the substance you need to find out.
Step 1:
Find the MOLES of the KNOWN substance. You can do this by DIVIDING the mass by the Mr of the KNOWN substance.
Step 2:
Here you need to look at the BIG numbers in front of the substances in the BALANCED equation.
You need to use the number IN FRONT of the KNOWN and UNKOWN to write the RATIO of them.
Known : Unknown
Use this ratio to find the MOLES of the UNKNOWN.
Step 3:
Find the mass by MULTIPLYING the MOLES of the UNKNOWN by its Mr.
Let's go through the steps using an example:
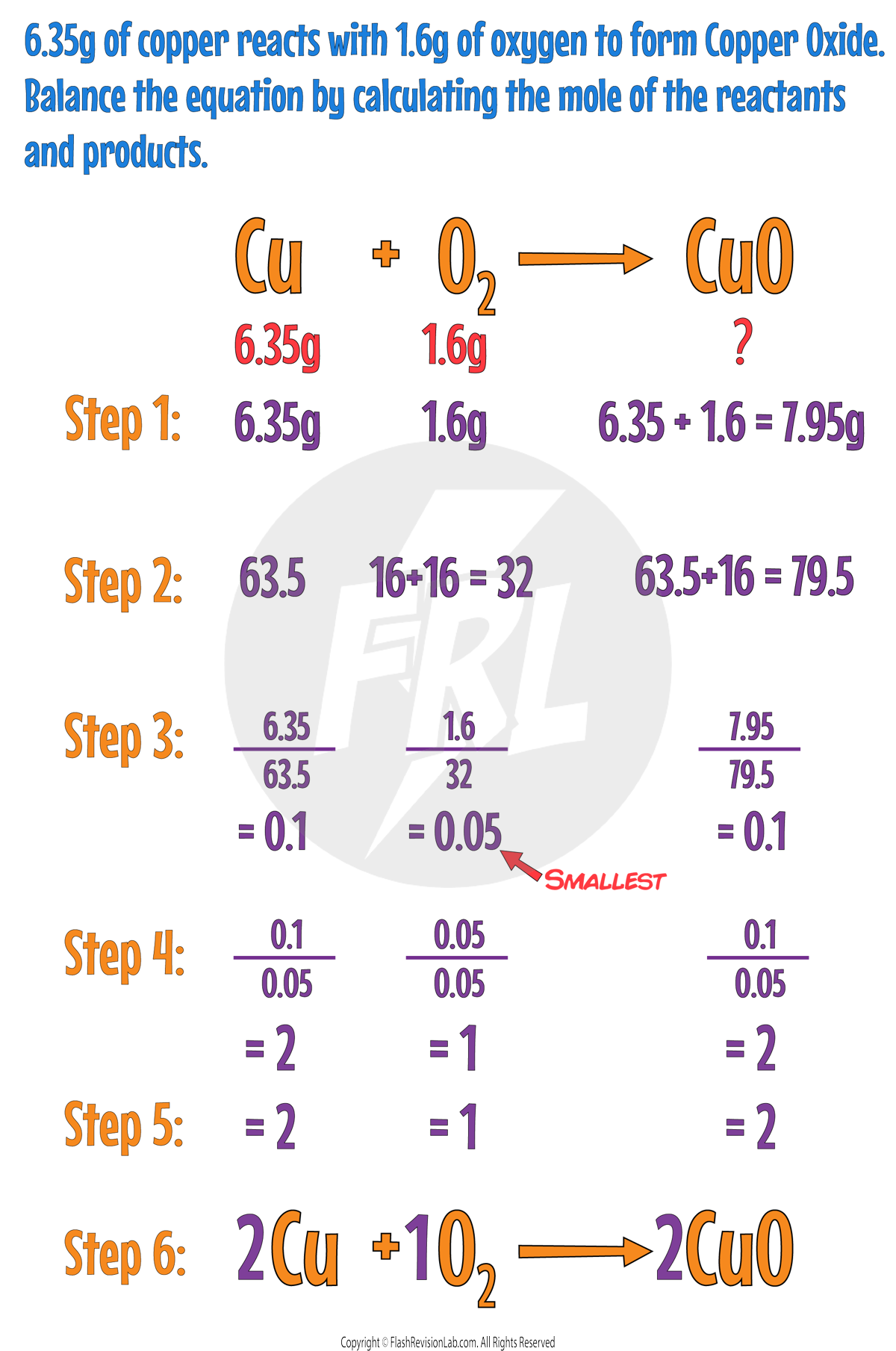
Using Moles to Balance Equations
If you know the masses of the REACTANTS and PRODUCTS that took part in a reaction, you can work out the BALANCED SYMBOL EQUATION for it.
You can do this by following these steps:
Step 1:
Find any unknown masses by using the CONSERVATION OF MASS.
Step 2:
Find the Mr for each reactant and product.
Step 3:
Divide the masses by the Mr for each reactant and product to find their MOLES.
Step 4:
Divide each of the moles by the SMALLEST NUMBER out of all of them.
Step 5:
If any of the values are NOT whole numbers, multiply all of them by a number that causes them to become WHOLE NUMBERS.
Step 6:
Put each of these numbers IN FRONT of their chemical formulas in the equation.
Example:
Limiting Reactants
In chemical reactions, you can have an EXCESS reactant and a LIMITING reactant.
When you have an EXCESS of a reactant, you have MORE of it than needed for the reaction. They are used to ensure another reactant is completely used up.
The LIMITING REACTANT is the substance that is COMPLETELY USED UP before the EXCESS REACTANT.
When the LIMITING REACTANT gets used up, the reaction STOPS meaning the EXCESS REACTANT would remain without reacting.
The AMOUNT OF PRODUCT that can be formed depends on the AMOUNT OF LIMITING REACTANT.
When performing reacting mass calculations, the LIMITING REAGENT is always the number that should be used, as it indicates the maximum possible amount of product that can form.
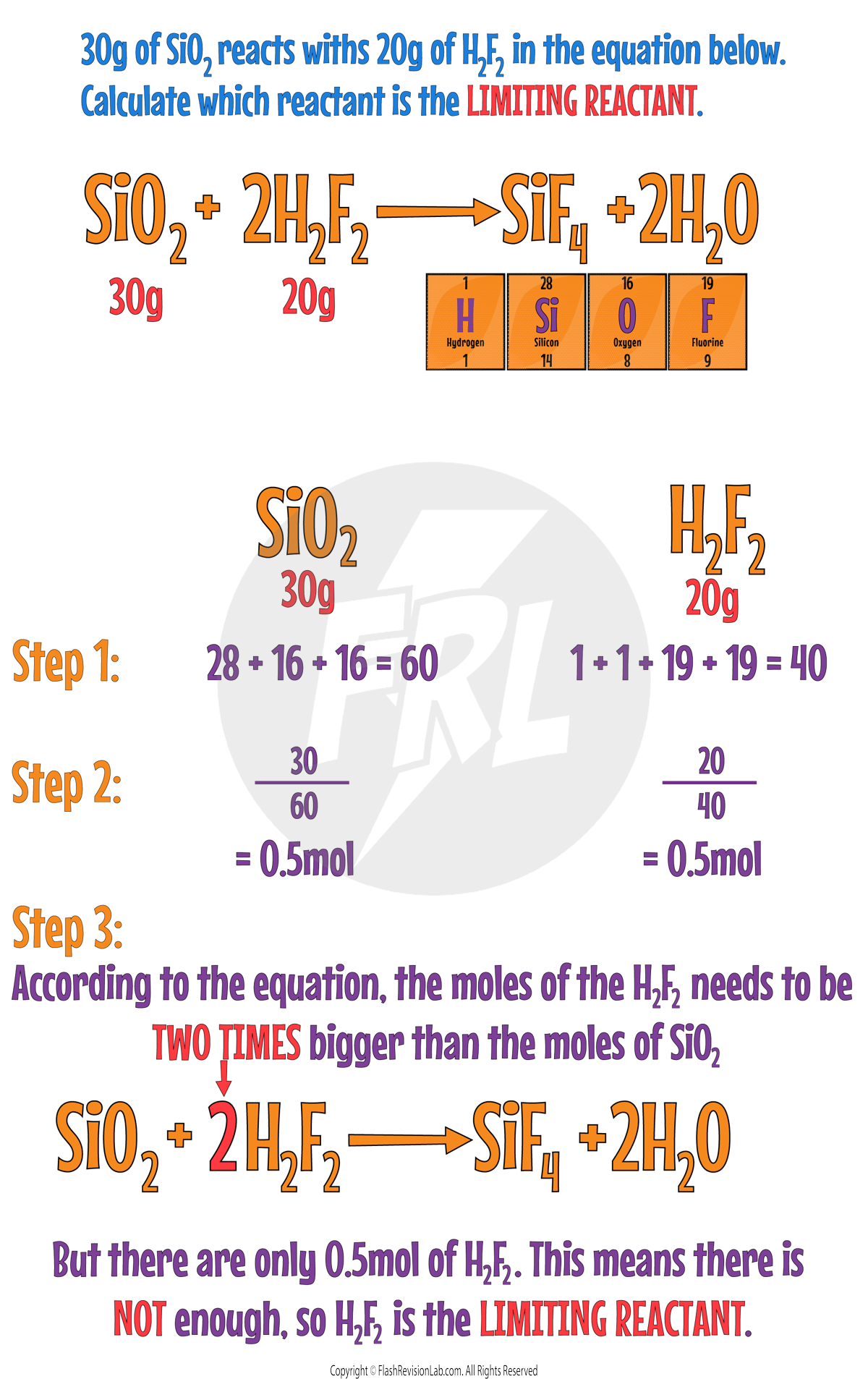
Determining the Limiting Reactant
In order to determine which reactant is the limiting reagent in a reaction, you have to use the AMOUNTS of each reactant used and the MOLAR RATIO of the balanced chemical equation.
To calculate the limiting reactant:
Step 1:
Find the Mr of each reactant.
Step 2:
CONVERT the mass of each reactant into MOLES by dividing by the Mr.
Step 3:
Compare the moles with the BALANCED EQUATION to see whether the moles match the ratio.
Let's use these steps in an example:

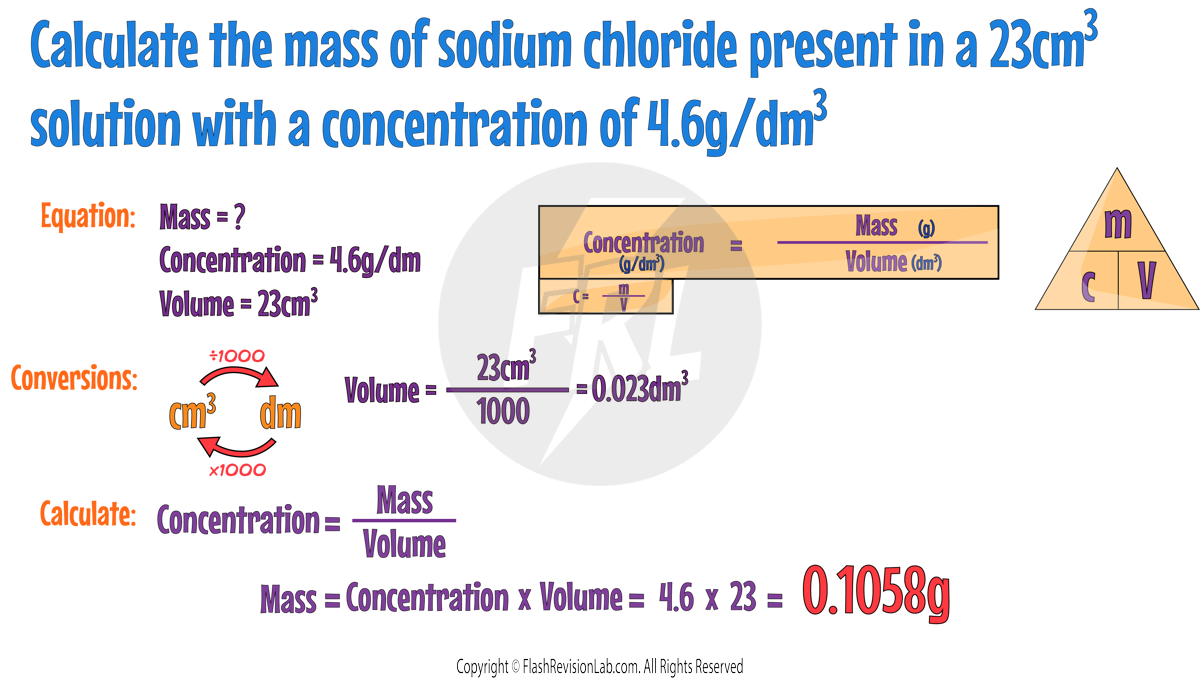
Concentration of Solutions
Understanding Concentration
A solid substance that dissolves in a liquid is called a SOLUTE.
The liquid it dissolves in is called a SOLVENT.
The mixture that a SOLUTE and SOLVENT form is called a SOLUTION.
CONCENTRATION is the AMOUNT OF A SUBSTANCE within a certain VOLUME of solution.
The more SOLUTE there is in a given volume, the MORE CONCENTRATED the solution is.
Measuring Concentration
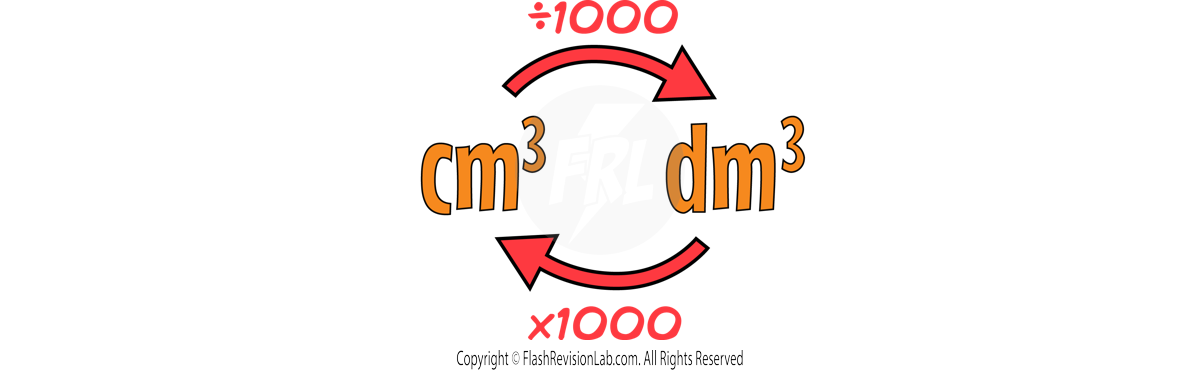
Volume can be measured in cm3 or dm3.
You can CONVERT between the two by multiplying and dividing by 1000:
Concentration is typically measured in grams per decimetre cubed (g/dm³).
You can calculate it using the formula:

Calculating Mass of Solute
If you know the CONCENTRATION and the VOLUME, you can calculate the MASS OF SOLUTE using the rearranged formula.
Here's an example:
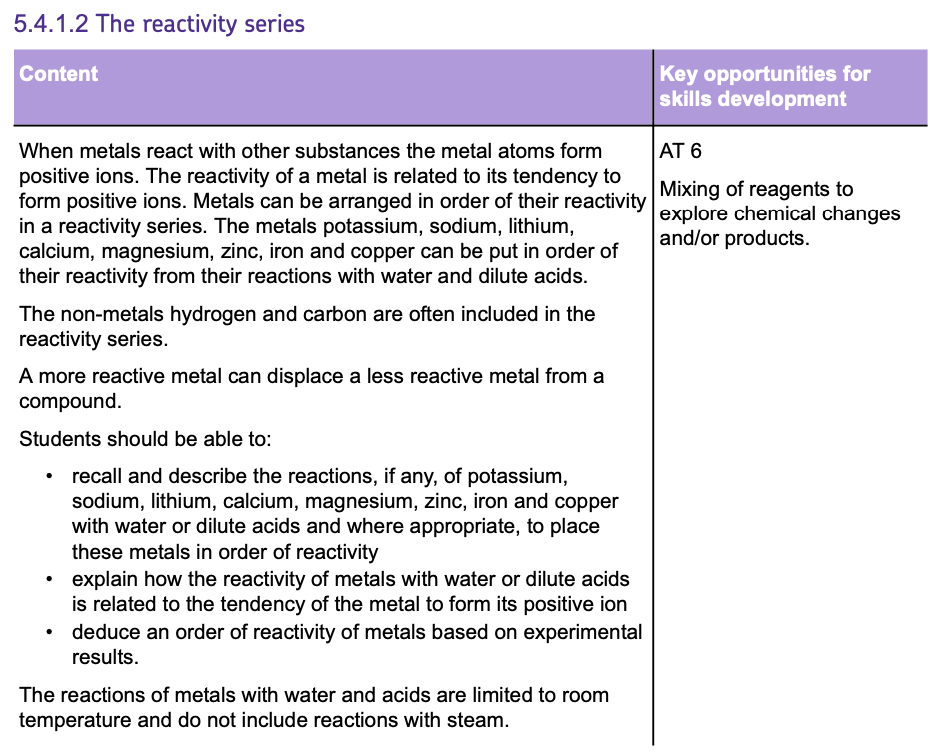
The Reactivity Series
Understanding the REACTIVITY SERIES helps predict how different metals will react with a variety of substances, including acids and water.
The Order of Reactivity
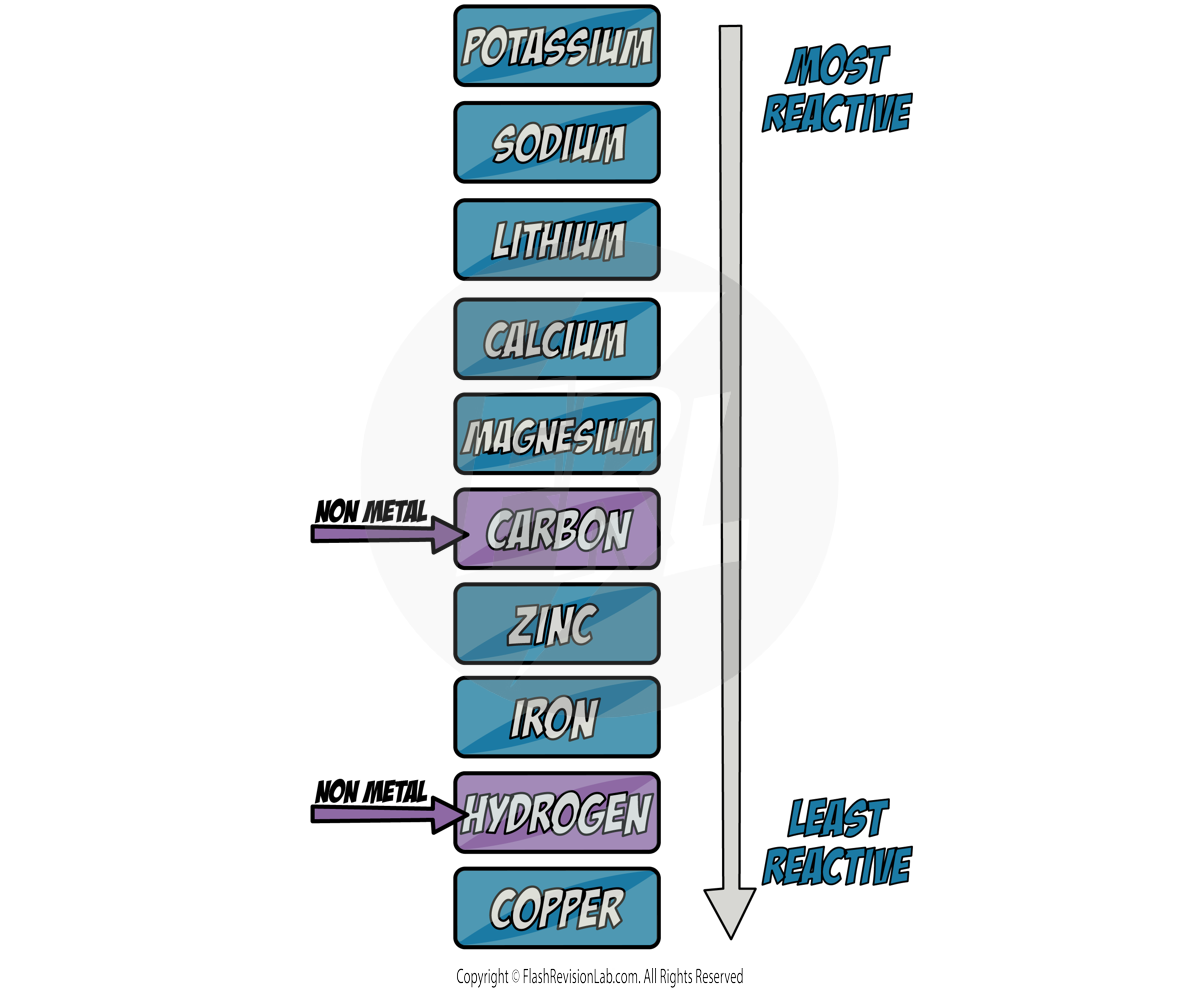
- The series ranks metals from the most reactive to the least based on how easily they lose electrons and form positive ions (cations).
- The MORE EASILY a metal LOSES electrons and forms POSITIVE IONS, the MORE REACTIVE it is.
Reactivity with Water and Acids
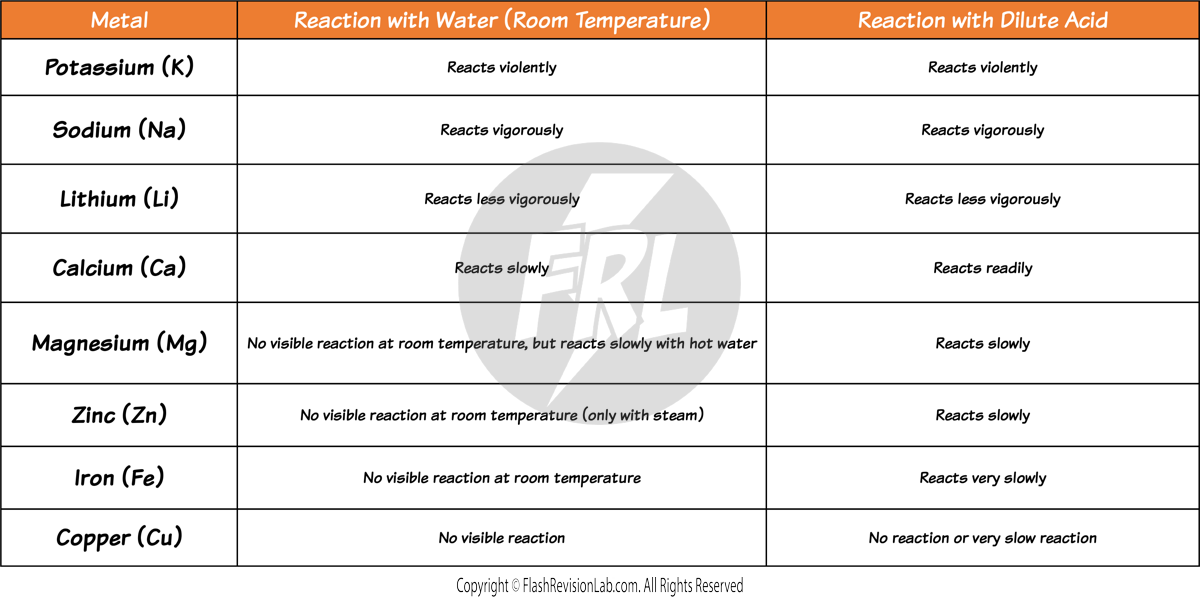
- You can find out how reactive a metal is by observing its reaction with WATER and ACID.
- The MORE VIOLENT the reaction, the MORE REACTIVE the metal.
- Highly reactive metals like POTASSIUM (K), SODIUM (Na), and LITHIUM (Li) react the most vigorously with water and acid, producing hydrogen gas.
- This reaction can be EXPLOSIVE with these metals due to their high reactivity.
- Metals like MAGNESIUM (Mg) and CALCIUM (Ca) also react with water and acid but LESS vigorously than the metals higher up in the series.
- ZINC (Zn) and IRON (Fe) do NOT react with liquid water, instead the water needs to be in the form of STEAM in order to react. Which shows that they have an even LOWER reactivity. They react with acid very slowly in comparison to the metals above.
- COPPER (Cu), is the only one that does not react with water OR acids, showing that it is at the bottom of this reactivity series.
Reaction Equations: Metal and Water
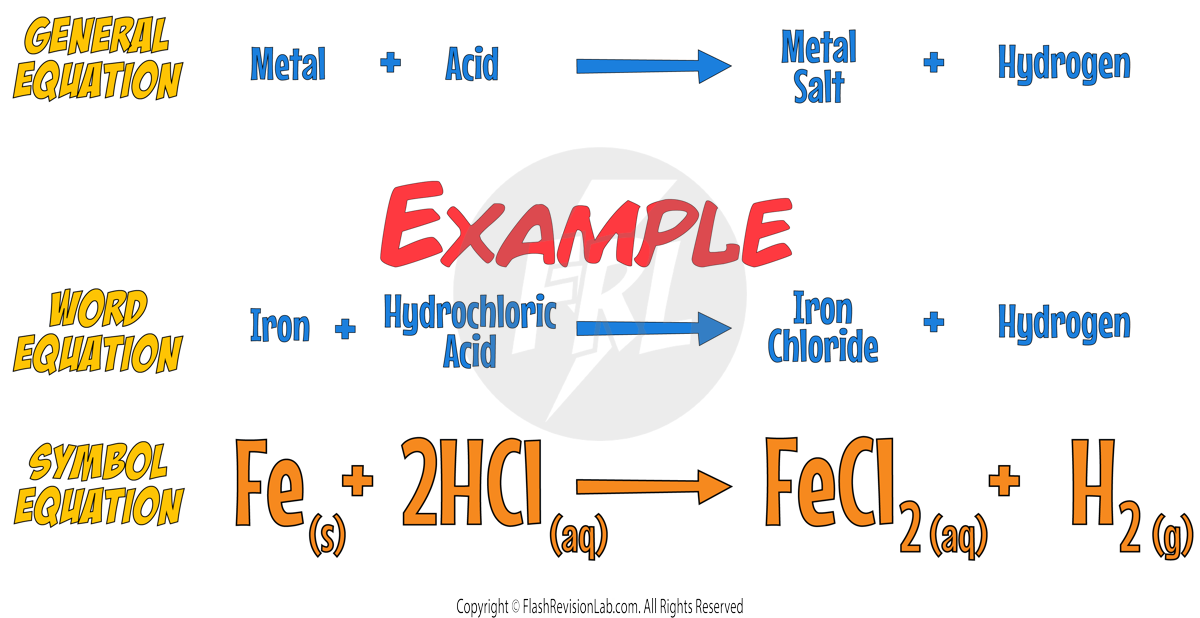
Reaction Equations: Metal and Acids
Metal Extraction
UNDERSTANDING METAL OXIDES
Metals combine with oxygen to form METAL OXIDES.

Most metals are found as METAL OXIDES in the environment in rocks called ORES, so they require chemical reactions to extract the PURE METAL from it.
OXIDATION AND REDUCTION

OXIDATION:
When metals like Iron or Aluminum react with Oxygen, they form metal oxides. This is known as oxidation, a chemical process involving the GAIN OF OXYGEN or the LOSS OF ELECTRONS.
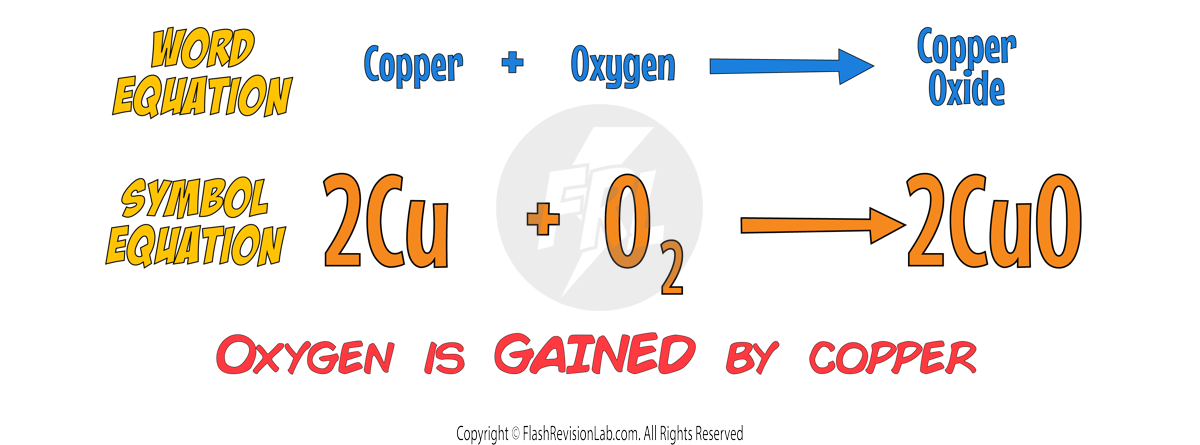
REDUCTION:
The process of EXTRACTING a metal from its oxide is known as a reduction reaction, because it involves the LOSS OF OXYGEN or the GAIN OF ELECTRONS.
EXTRACTION TECHNIQUES
There are THREE methods of getting METALS from the Earth. The method that should be used is based on the metal's position in the reactivity series:
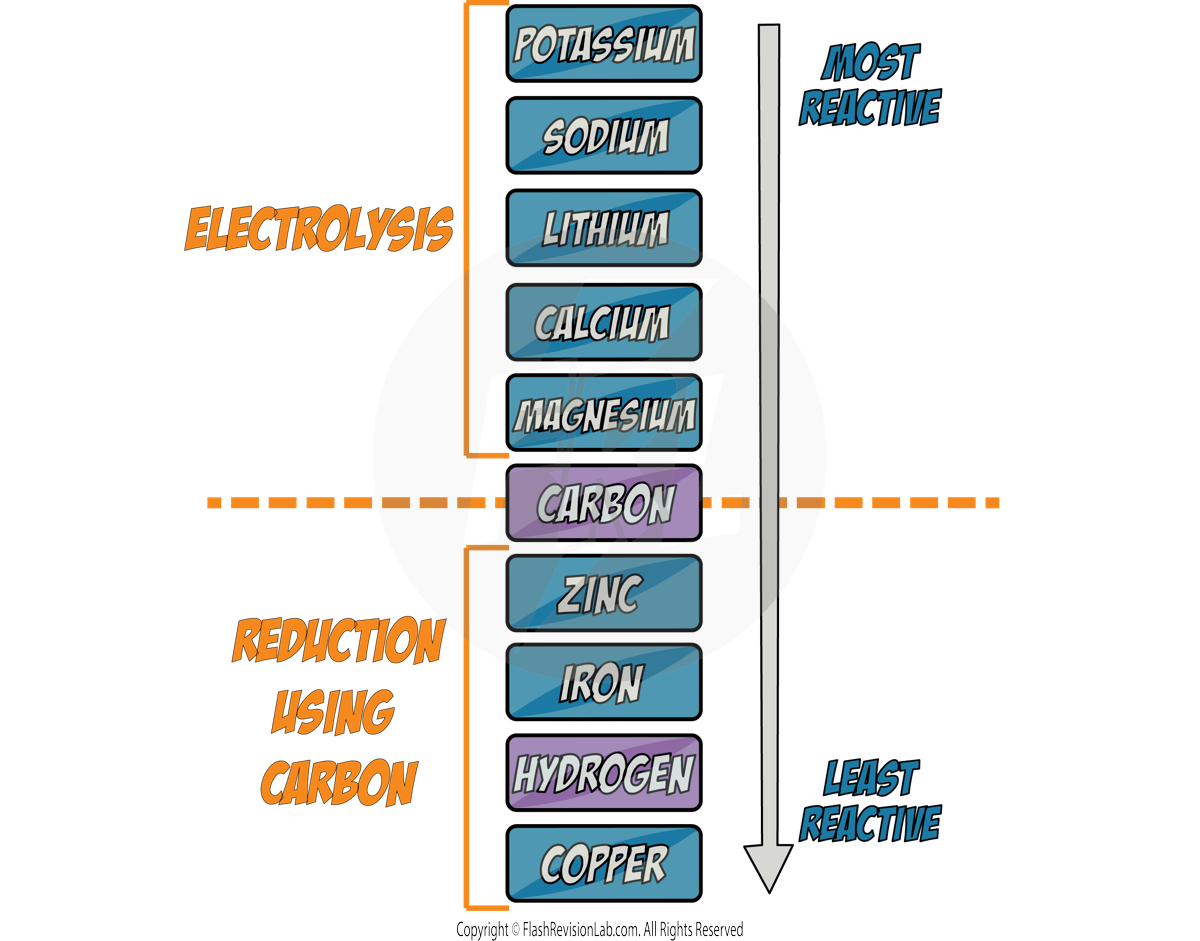
1. No Extraction
Metals that are UNREACTIVE like GOLD can be found on Earth as itself, so NO extraction is required. It can simply be collected.
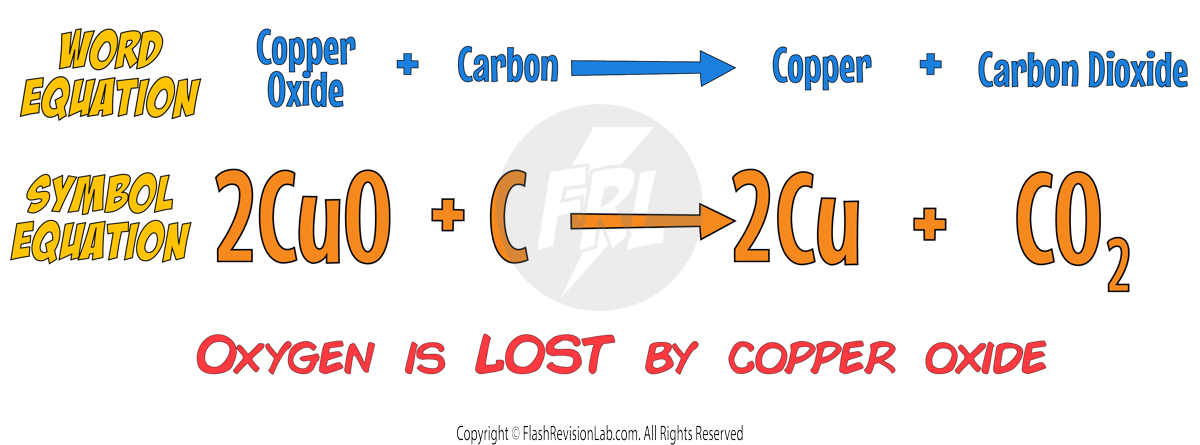
2. Reduction with Carbon
Metals LESS reactive than CARBON can be extracted using REDUCTION.
With these metals, carbon will always be MORE REACTIVE than the metal so it will REMOVE the oxygen from the metal and form CARBON DIOXIDE.
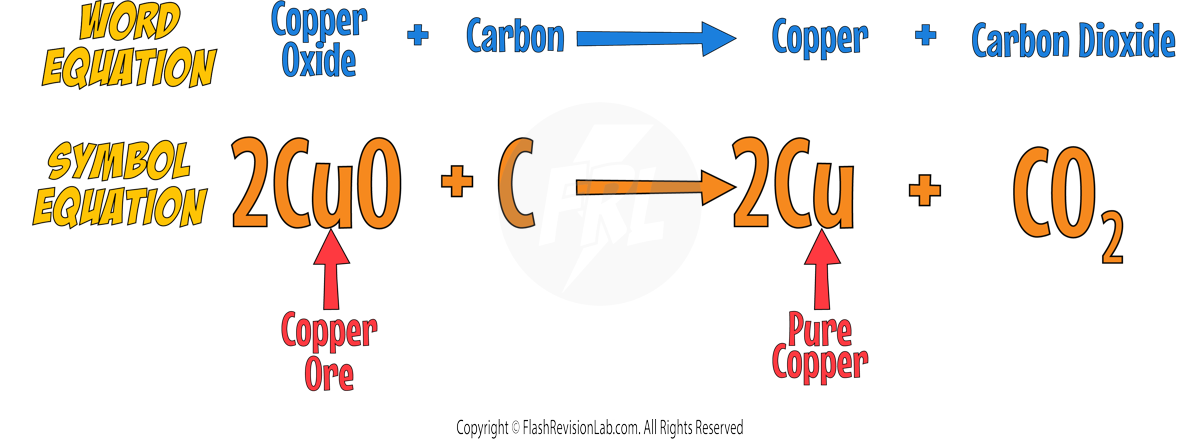
3. Electrolysis
Metals MORE reactive than CARBON will NOT be REDUCED by it. So ELECTROLYSIS is used instead which is a MORE EXPENSIVE method due to the cost of electricity. (see later notes)
Redox Reactions and Half Equations
What is Redox?
- REDOX stands for reduction-oxidation. It is when reduction AND oxidation occur in the same reaction.
- These reactions are all about the transfer of ELECTRONS between substances.
- OXIDATION is the process where a substance LOSES ELECTRONS. This often involves the addition of oxygen.
- REDUCTION is the process where a substance GAINS ELECTRONS, typically losing oxygen.
Remember: OIL RIG – "Oxidation Is Loss, Reduction Is Gain."

This helps you remember what happens to electrons during redox reactions.
Displacement Reactions:
DISPLACEMENT REACTIONS are examples of REDOX REACTIONS.
These occur when a MORE REACTIVE METAL replaces a LESS REACTIVE METAL in a compound.
For instance, when Zinc is placed in a Copper Sulfate solution:
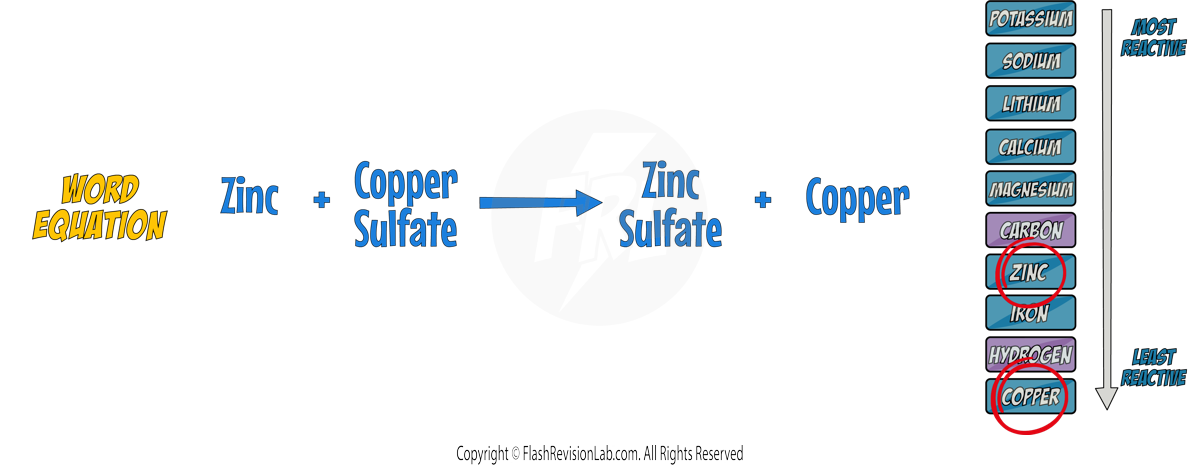
Zinc is HIGHER in the reactivity series so it REPLACES copper to form ZINC SULFATE.
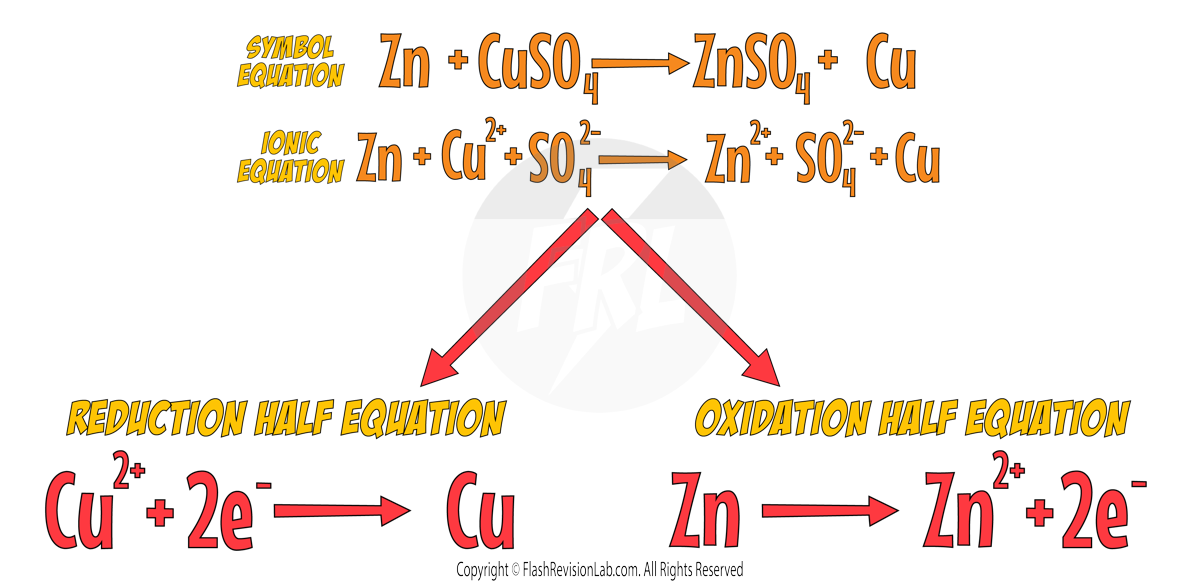
To show that this is a redox reaction, let's look at the SYMBOL EQUATION.

We can now break up the symbol equation to form the IONIC EQUATION, which shows all of the ions involved in the reaction.
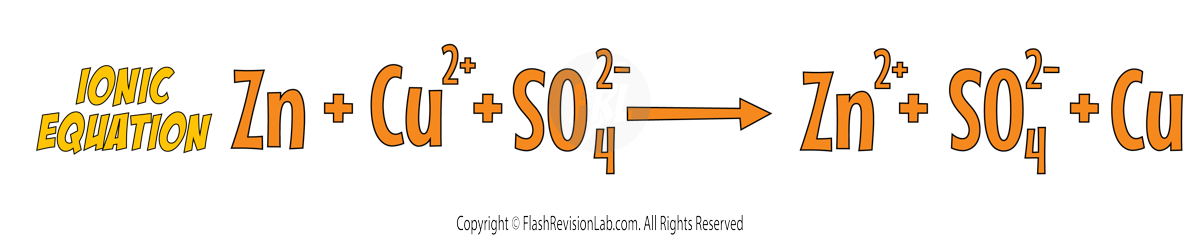
If we look at just the element ZINC in this equation we can see that Zn turns into Zn2+.
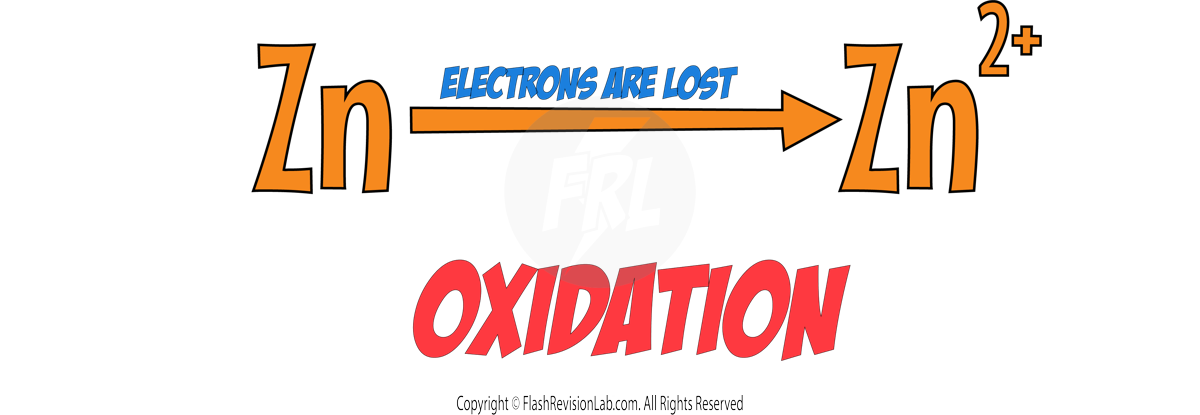
To get a charge of +2 the Zinc must have LOST TWO ELECTRONS. Using OIL RIG, this tells us that Zinc has been OXIDISED.
Now let's look at the element COPPER:
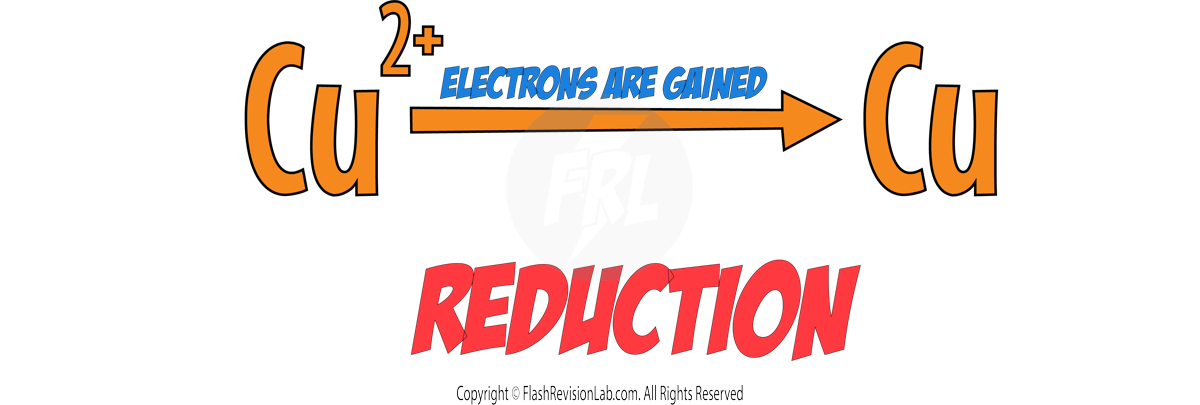
To go from Cu2+ to Cu, Cu2+ has GAINED two electrons. So we can say Copper has been REDUCED.
WRITING HALF EQUATIONS
Half Equations show the ELECTRON TRANSFERS within an ionic equation. Electrons in half equations are represented as an e-.
You can think of them as HALF of an ionic equation and there are always TWO:
1. The REDUCTION half equation
This shows the element that GAINS electrons. Electrons in this type are written on the REACTANTS side (left hand side).
2.The OXIDATION half equation
This shows the element that LOSES electrons. Electrons in this type are written on the PRODUCTS side (right hand side).
Let's see how the half equation looks for Zinc reacting with Copper Sulfate:
The pH Scale and Neutralisation
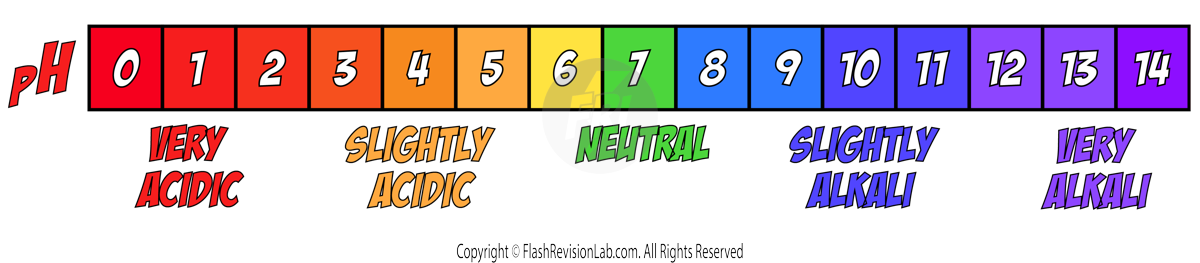
- pH is a measurement that tells you how ACIDIC or ALKALI an aqueous solution is.
- The pH scale goes from 0-14, and the HIGHER the pH value, the MORE ALKALI a solution is.
- ACIDS release HYDROGEN IONS (H+) in solution and have a pH less than 7.
- ALKALIS release hydroxide ions (OH⁻) and have a pH greater than 7.
- NEUTRAL SOLUTIONS have a pH of exactly 7, indicating a balance between H⁺ and OH⁻ ions.
How to Measure pH
Using Indicators:
- INDICATORS are dyes that show different colours at different pH levels.
- WIDE-RANGE INDICATORS: These can show a VARIETY of colours across the pH scale, allowing you to make a rough estimate of pH. An example of a WIDE RANGE INDICATOR is UNIVERSAL INDICATOR which can give the following colours:
Using pH probes:
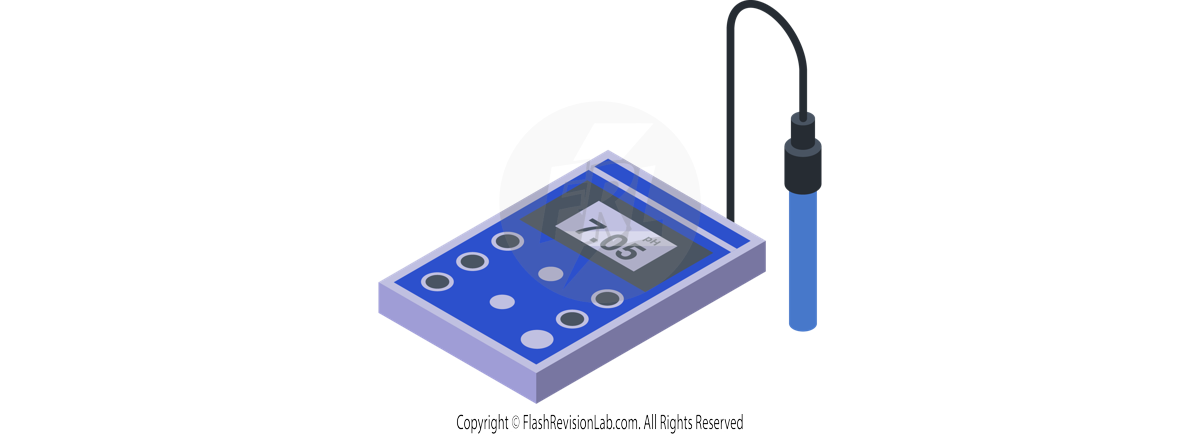
- pH PROBES give a DIGITAL reading of pH, offering a more PRECISE measurement.
- The pH probe is immersed in the solution, and the meter gives a reading for the pH values.
Neutralisation
NEUTRALISATION is a type of chemical reaction in which an acid and a base react to form water and a salt.

You can also write the IONIC EQUATION for ANY neutralisation reaction like this:
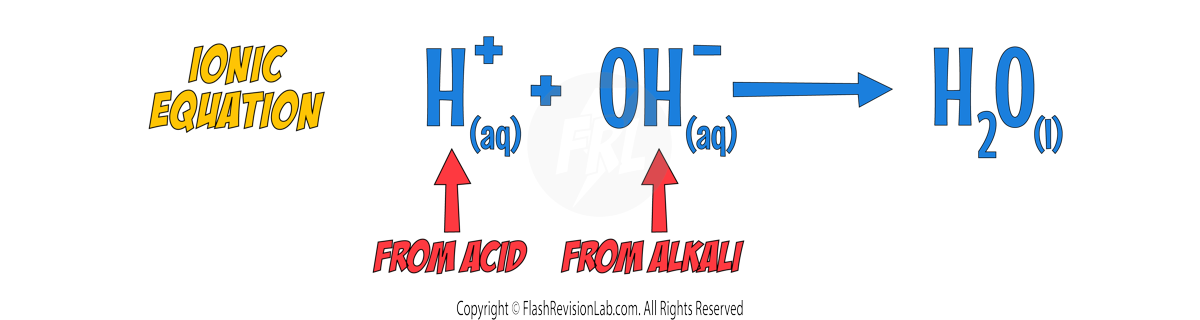
This reaction involves the transfer of H⁺ IONS from the acid to the OH⁻ IONS of the alkali, forming H₂O.
Strong and Weak Acids
HOW ACIDS WORK
ACIDS are solutions that release HYDROGEN IONS (H⁺) in water.
They do this because they IONISE when dissolved in water. This means the molecules of the acid BREAK UP into their IONS.
For example:
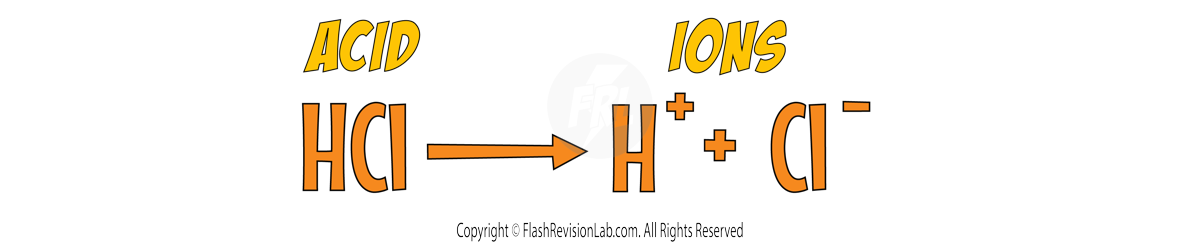
STRONG vs. WEAK ACIDS
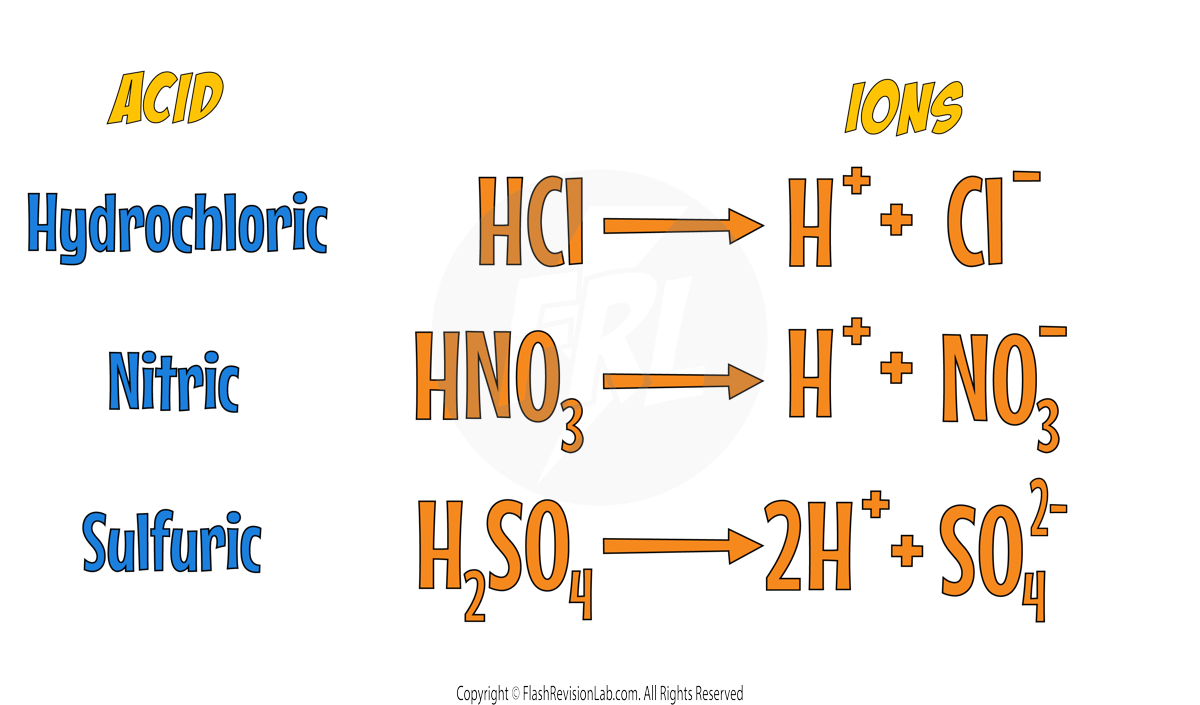
STRONG ACIDS:
These are acids that FULLY IONISE in water, meaning ALL of the acid molecules break up into IONS.
This means ALL their available H+ ions are released into the solution.
This complete ionisation leads to a HIGHER CONCENTRATION OF H⁺ IONS, which generally results in a LOWER pH.
Here are some common STRONG ACIDS and their ionising equations:
WEAK ACIDS:
These acids PARTIALLY IONISE in water, which means only SOME of the acid molecules break up into their ions.
The incomplete release of H⁺ ions results in a LOWER CONCENTRATION OF H+ IONS, which generally results in a HIGHER pH compared to strong acids of the same concentration.
Examples of WEAK ACIDS include, ETHANOIC ACID, CITRIC ACID and CARBONIC ACID.
RELATIONSHIP BETWEEN pH AND H⁺ ION CONCENTRATION
The MORE H+ IONS there are in a give volume of solution the MORE ACIDIC the solution is.
This means the HIGHER the H+ CONCENTRATION of an acid, the LOWER the pH. The relationship between the two is:
If you INCREASE the H⁺ ION CONCENTRATION by a factor of 10 TIMES, the pH will DECREASE by 1.
You can extend this relationship to bigger changes in pH and H+ concentrations:
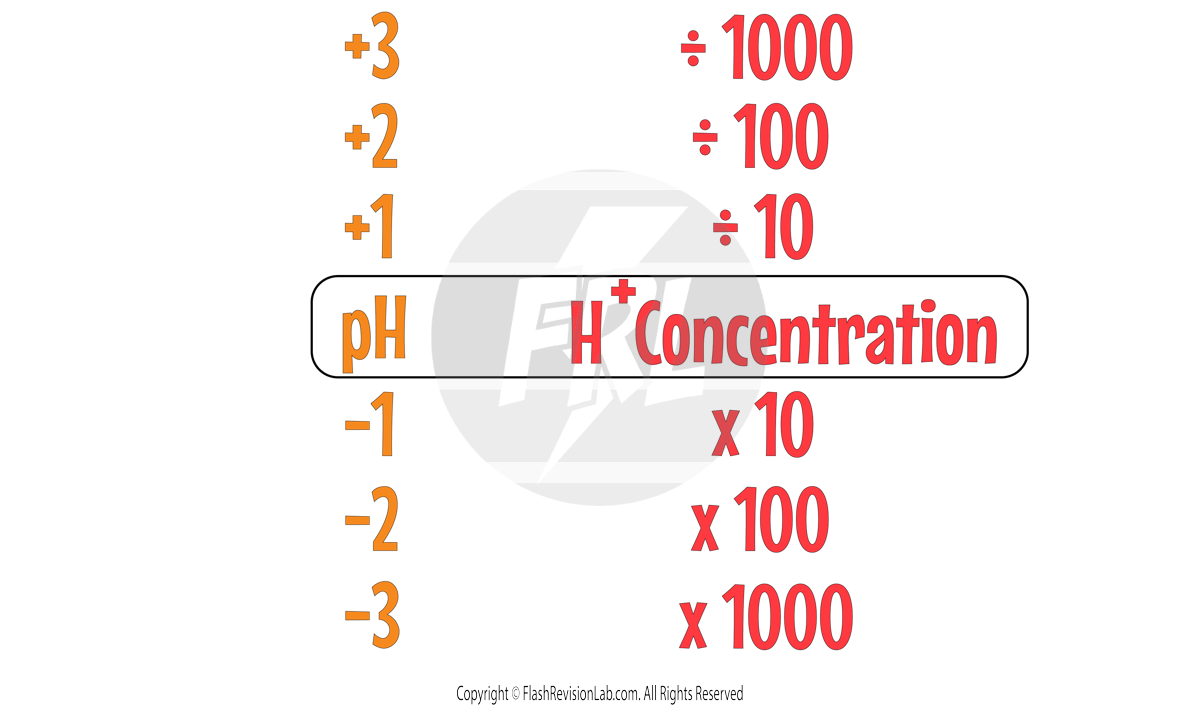
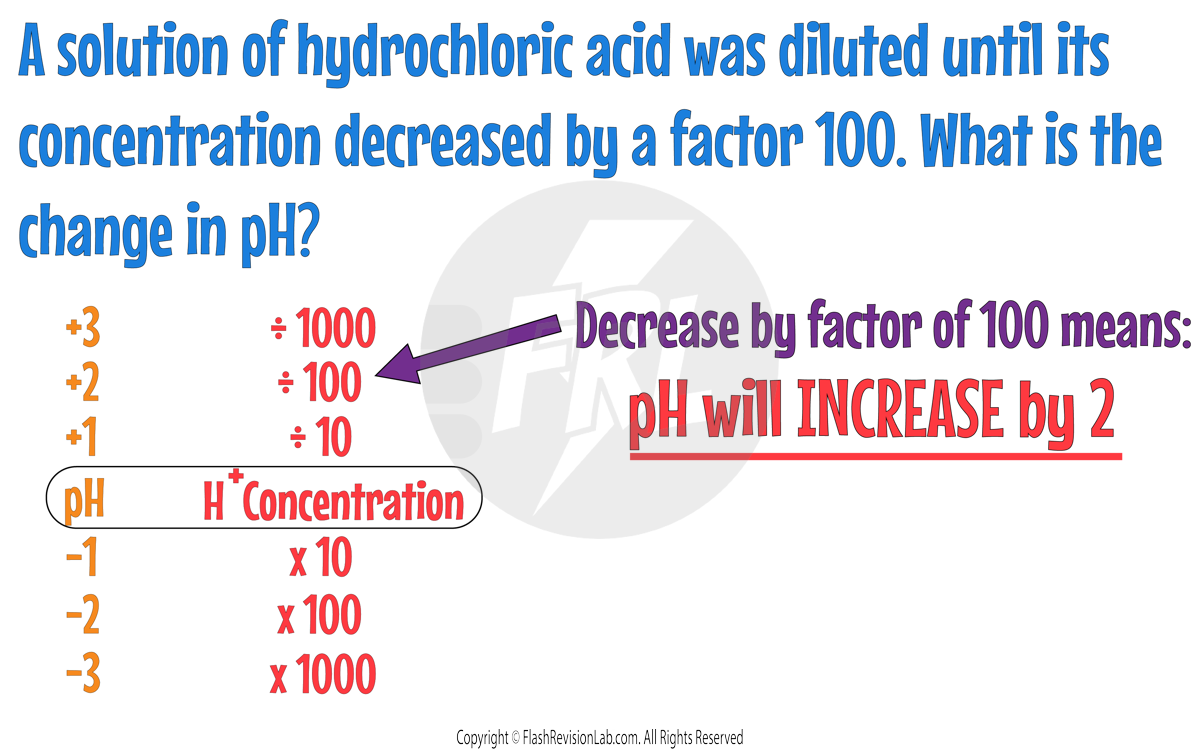
Let's try an example using this relationship:
ACID STRENGTH vs CONCENTRATION
Don’t get confused between the strength of an acid and its concentration:
- The CONCENTRATION of an acid tells you the NUMBER OF ACID MOLECULES in a given volume.
- The STRENGTH refers to the acid's degree of ionisation in water (how easily it breaks up into its ions).
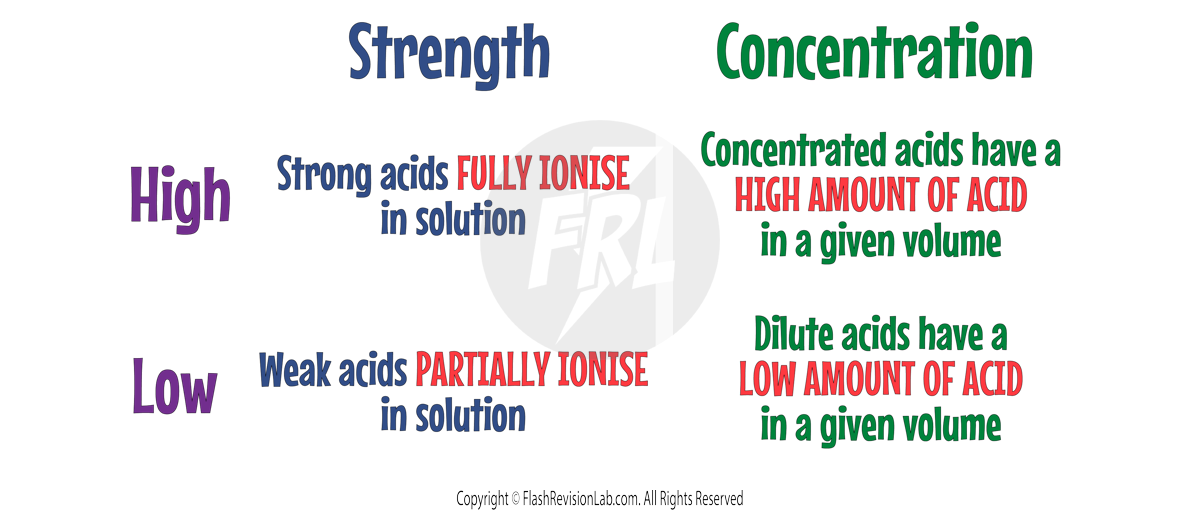
Concentration and strength are INDEPENDENT of each other, meaning you can have:
- A STRONG, CONCENTRATED Acid.
- A STRONG, DILUTE Acid.
- A WEAK, CONCENTRATED Acid.
- A WEAK, DILUTE Acid.
Reactions of Acids
METAL OXIDES and METAL HYDROXIDES are classified as BASES, which are typically substances that can NEUTRALISE ACIDS.
ALKALIS are BASES that are SOLUBLE in water (metal hydroxides).
These compounds react with ACIDS to form a SALT and WATER, in a process known as NEUTRALISATION.
The type of SALT formed depends on the acid used in the reaction:
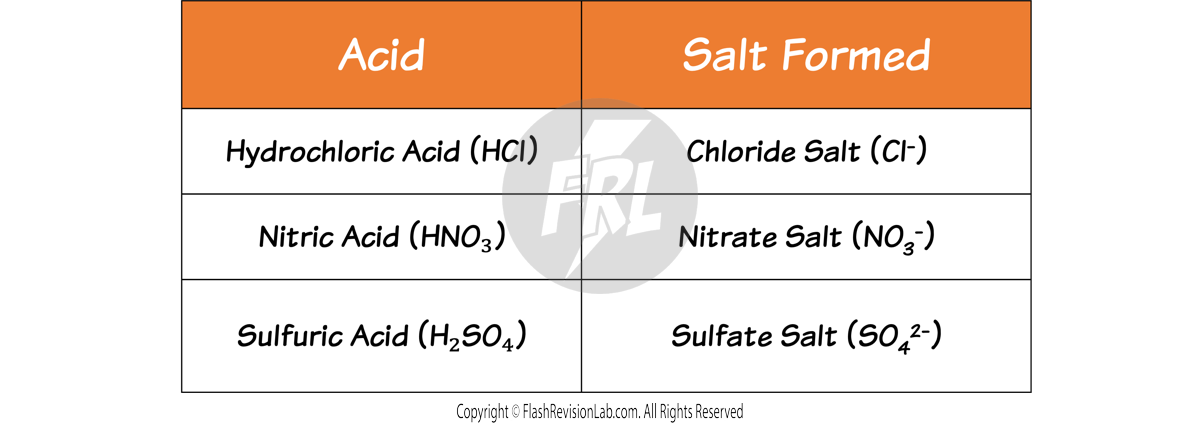
By knowing these, you can predict many equations for neutralisation reactions.
- When an acid reacts with a METAL OXIDE, the general equation is:
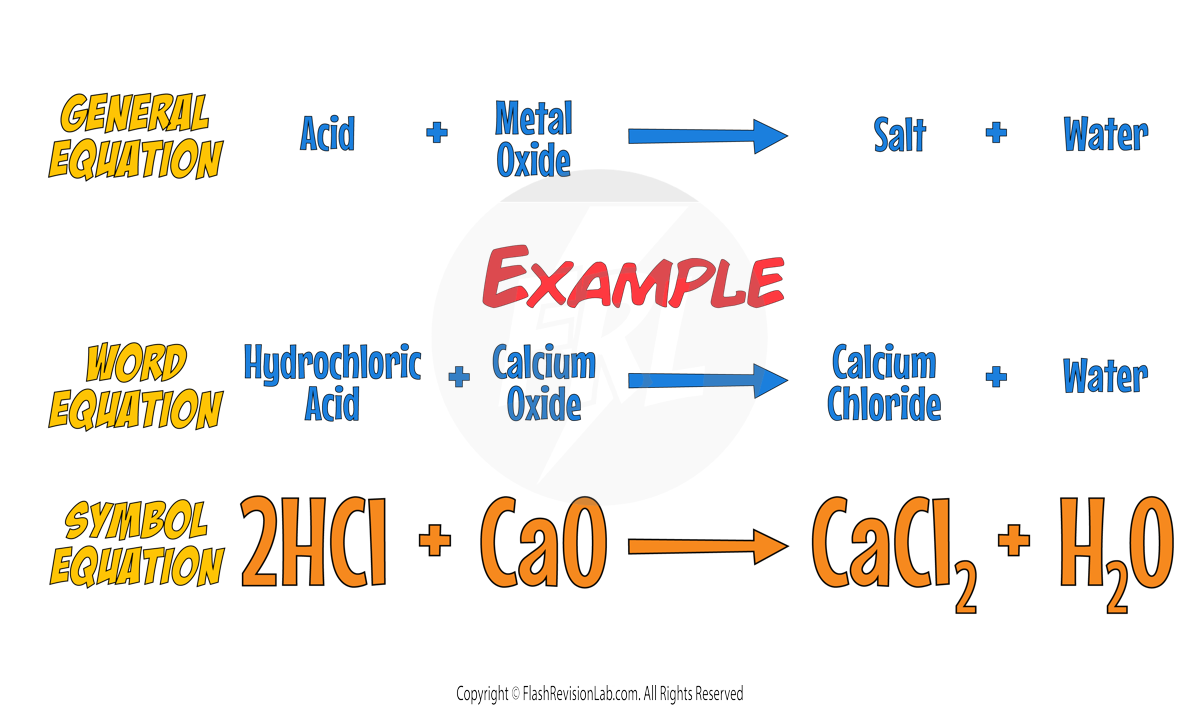
- Similarly, an acid's reaction with a METAL HYDROXIDE can be represented as:
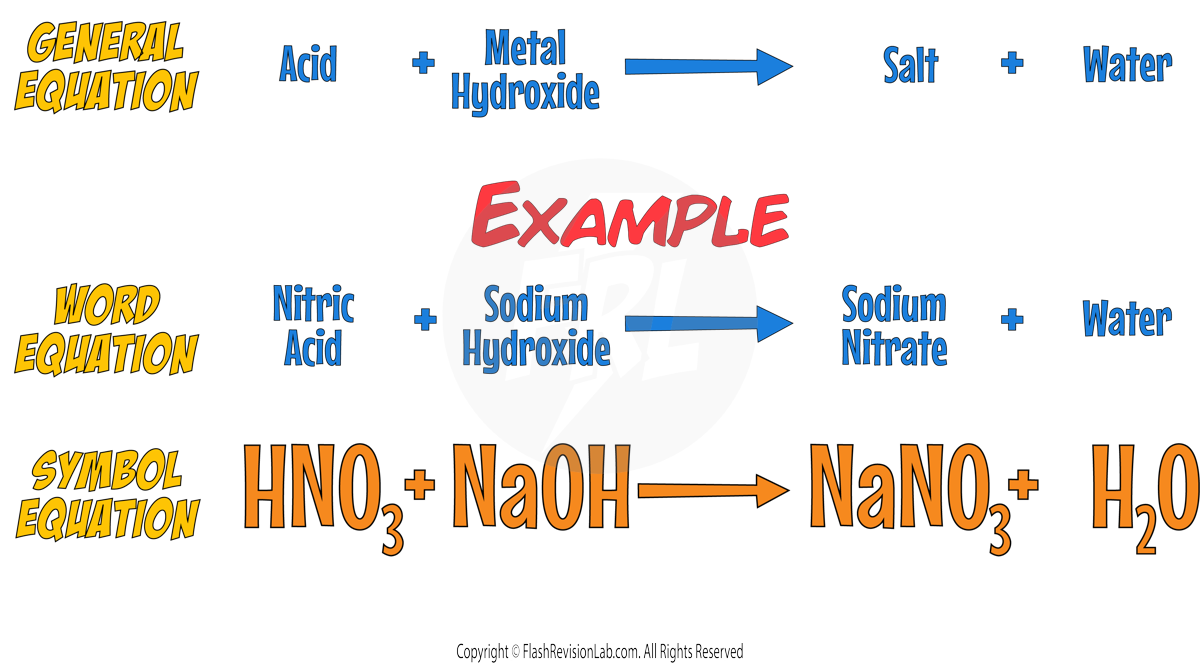
- METAL CARBONATES are also bases and their reaction with acids produces not only salt and water but also CARBON DIOXIDE gas.
- The general equation for this reaction is:
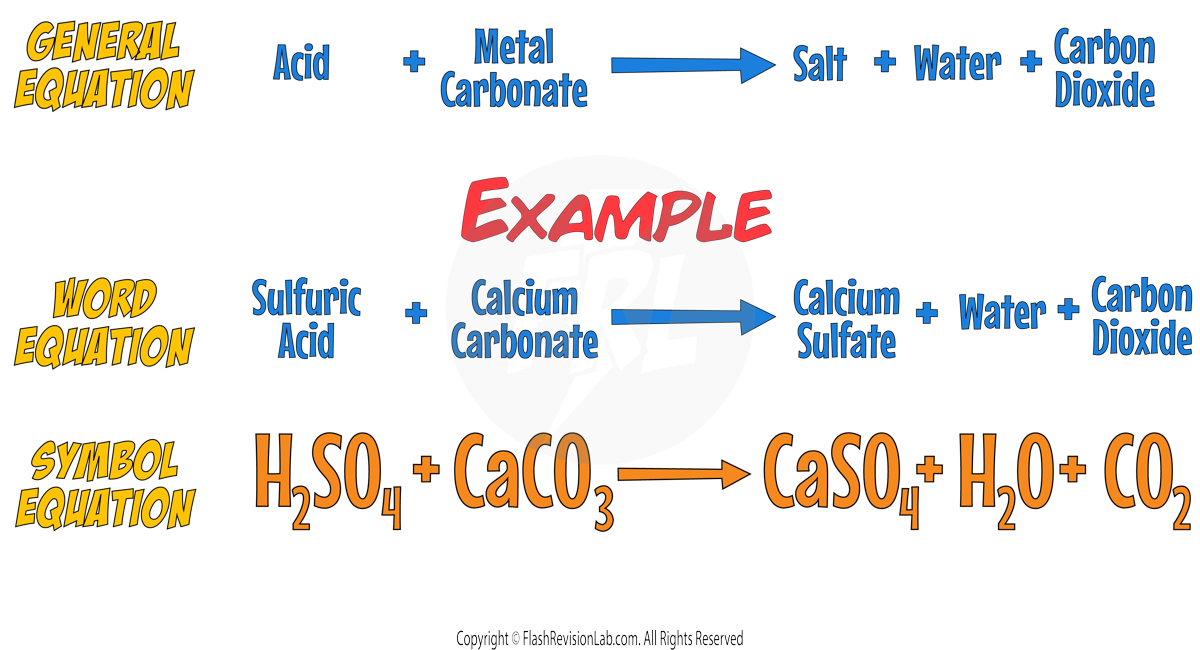

Required Practical: Making Salts
SALTS are often created by reacting an ACID with a BASE.
To make a SOLUBLE salt from an INSOLUBLE base, you need to use the correct reactants.
For example, if you wanted to make LITHIUM CHLORIDE. You would need to pick the correct BASE to give you the LITHIUM and the correct ACID to give you the CHLORIDE.
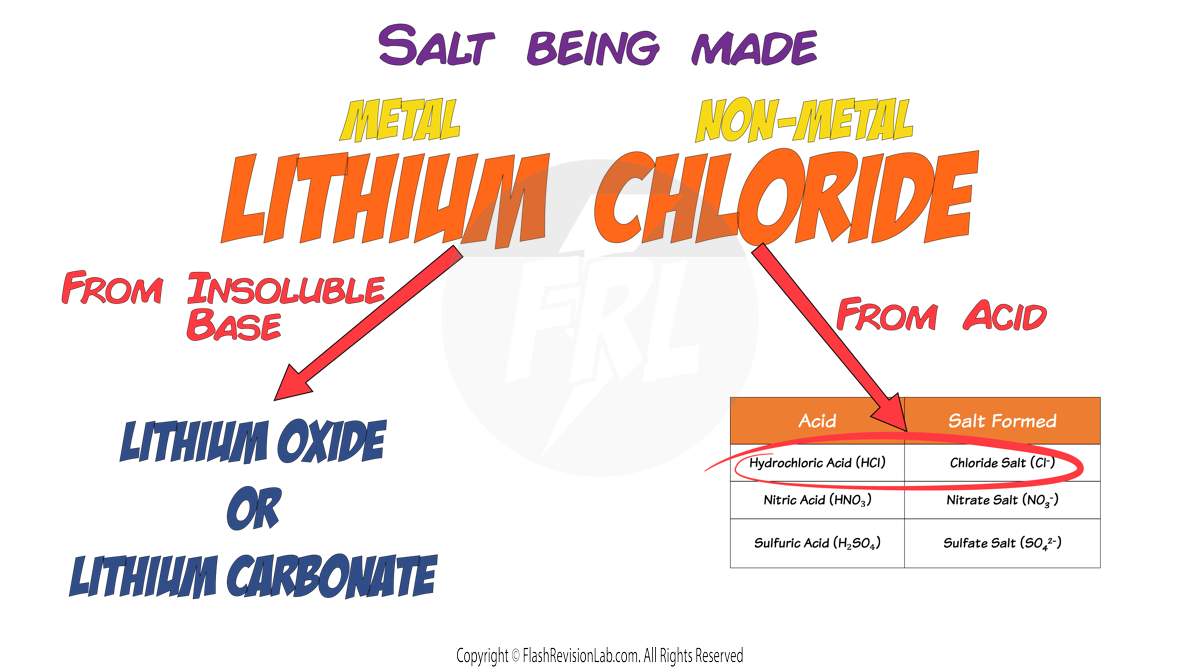
So to create a Lithium Chloride salt you can use the two possible combinations of reactants:
1. Lithium Oxide and Hydrochloric Acid
2. Lithium Carbonate and Hydrochloric Acid
Process of Making Salts
To make the salt, follow these steps:
You need to mix HYDROCHLORIC ACID (HCl) with LITHIUM OXIDE (LiO) in a beaker while the acid is warm:
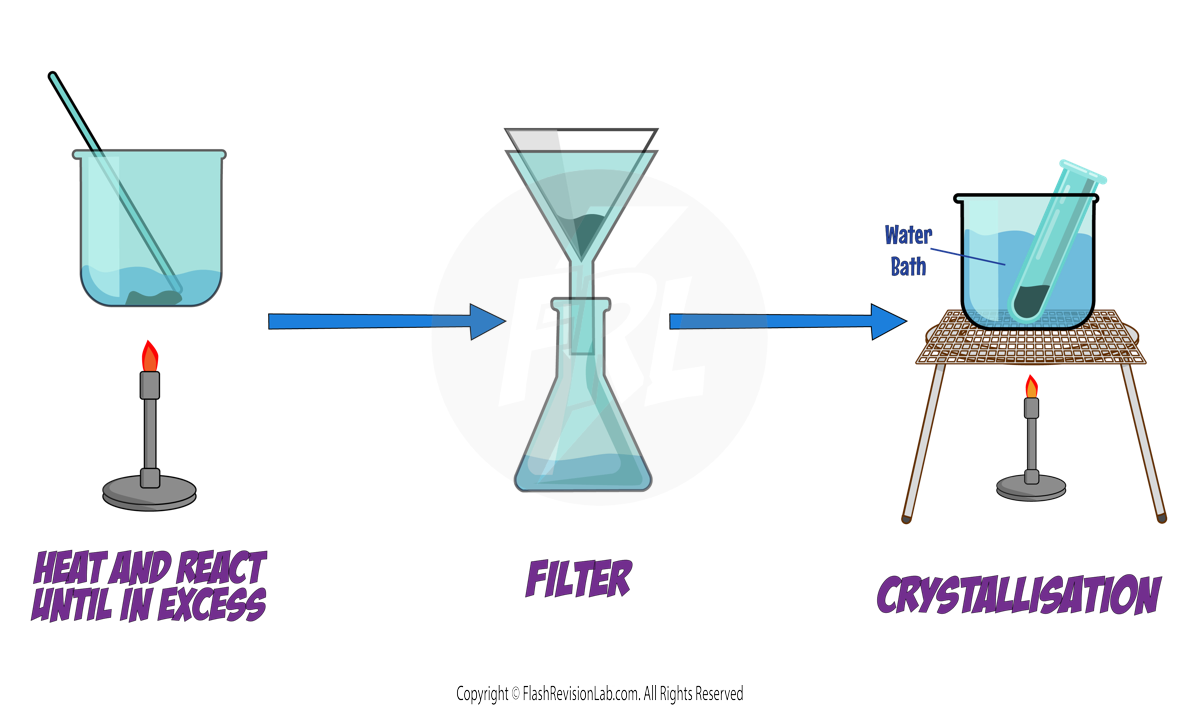
1. Heat the acid gently using a BUNSEN BURNER.
2. Add the LITHIUM OXIDE to the acid little by little while STIRRING. Continue until NO MORE DISSOLVES. At this point, the Lithium Oxide is in EXCESS and it will settle at the bottom of the beaker without reacting.
3. Remove the undissolved EXCESS Lithium Oxide by FILTRATION to collect the solution containing the salt.
4. To obtain pure, solid crystals of the salt, gently HEAT the solution using a WATER BATH to evaporate some water, increasing the solution's concentration. Once the solution is saturated, allow it to COOL. The salt will crystallise out and can be collected by filtration.

The Process of Electrolysis
ELECTROLYSIS is a chemical process where an ELECTRIC CURRENT is used to break up an IONIC COMPOUND.
The set up for electrolysis looks like this:
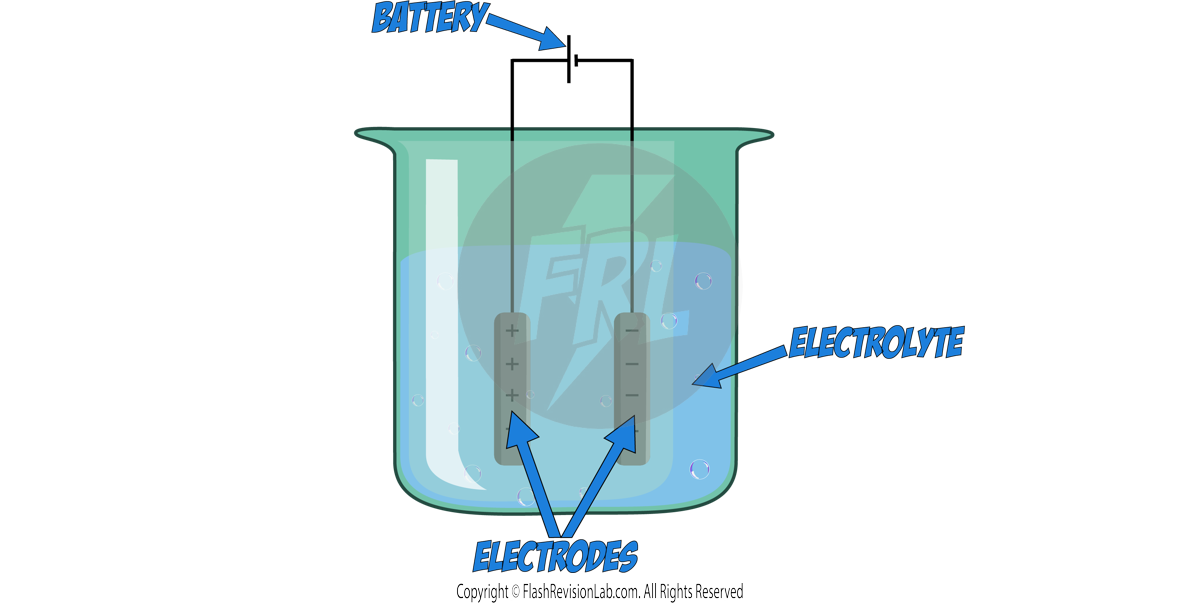
ELECTROLYTE:
This is the IONIC COMPOUND that is being separated.
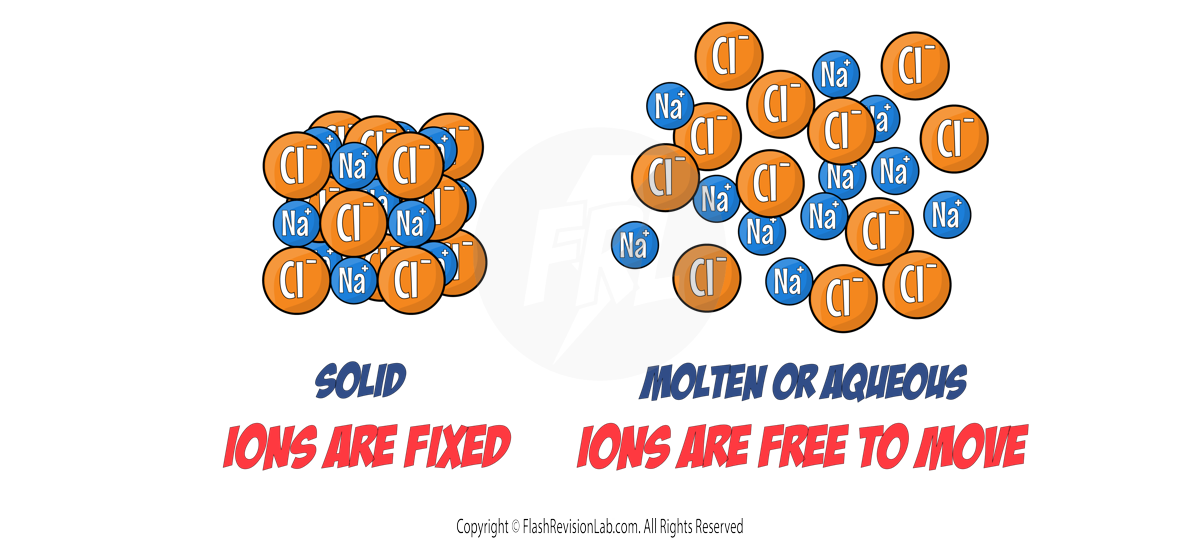
The ionic compound needs to be either MOLTEN (melted to liquid) or AQUEOUS (dissolved in water) so that it can CONDUCT ELECTRICITY. This is because when ionic compounds are SOLID, the ions are NOT free to move, but when they are molten or aqueous, they ARE free to move.
ELECTRODES:
To pass electricity through the electrolyte, a circuit with a battery is connected to Carbon electrodes which are submerged within the electrolyte.
There are TWO types of electrodes:
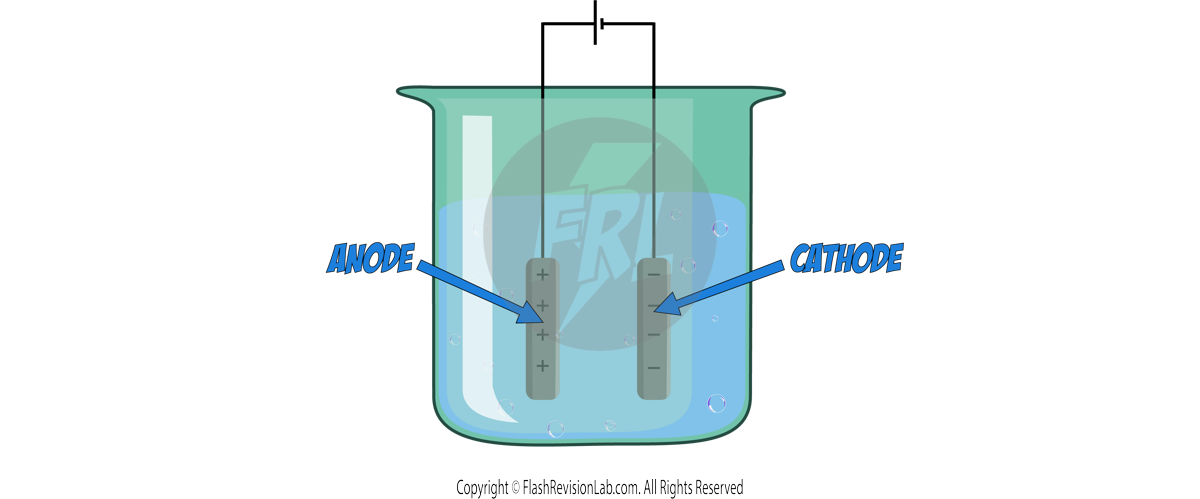
1. Anode: The POSITIVE electrode
2. Cathode: The NEGATIVE electrode
THE ELECTROLYSIS PROCESS
- When electricity passes through the electrolyte, the free moving IONS MOVE to the electrodes.
- POSITIVELY charged ions move to the CATHODE (negative) due to ATTRACTION of the OPPOSITE charges.
- Similarly, NEGATIVELY charged ions are attracted to the ANODE (positive).
- When the ions make contact with the electrodes, they DISCHARGE and turn into ATOMS of ELEMENTS.
- Different types of reactions occur at each electrode:

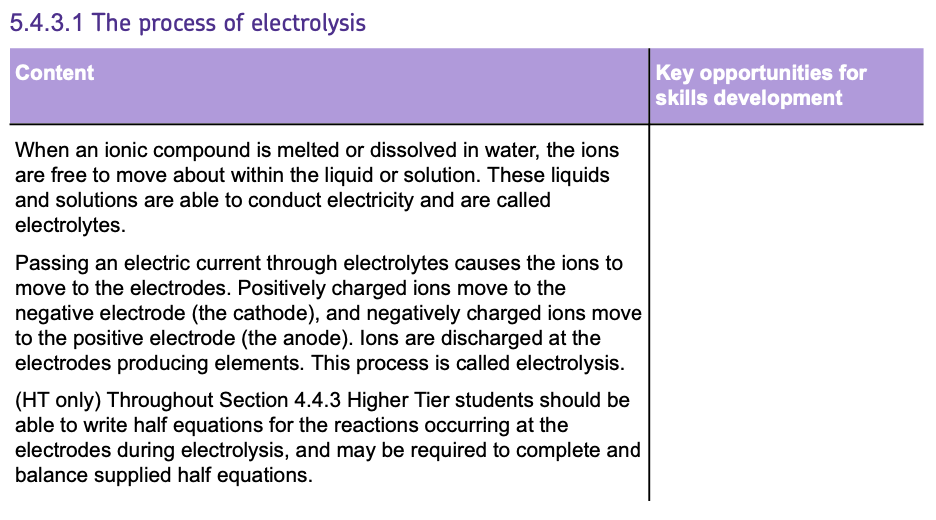
Electrolysis of Molten Ionic Compounds
- Ionic compounds in the molten state conduct electricity because their IONS are FREE TO MOVE.
- The PRODUCTS formed from MOLTEN electrolytes are ALWAYS the elements present in the ELECTROLYTE.
e.g. Electrolysis on MOLTEN Sodium Chloride will always form SODIUM and CHLORINE whereas AQUEOUS Sodium Chloride does not (see later notes).
Let’s look at the process for the example of MOLTEN SODIUM CHLORIDE electrolyte:
The IONS involve in this example are Na+ and Cl-. This means that the electrolyte has these ions floating around in it.
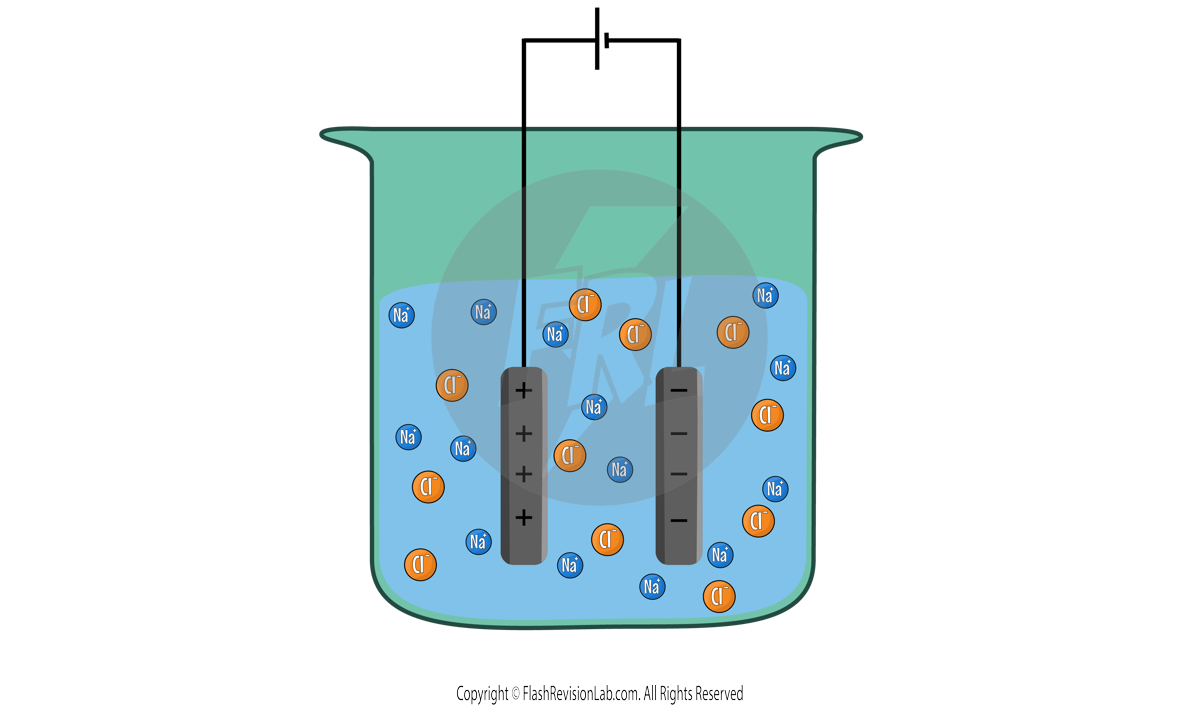
When the circuit is turned on, and electricity flows, the Na+ ions get attracted to the CATHODE and the Cl- ions get attracted to the ANODE.
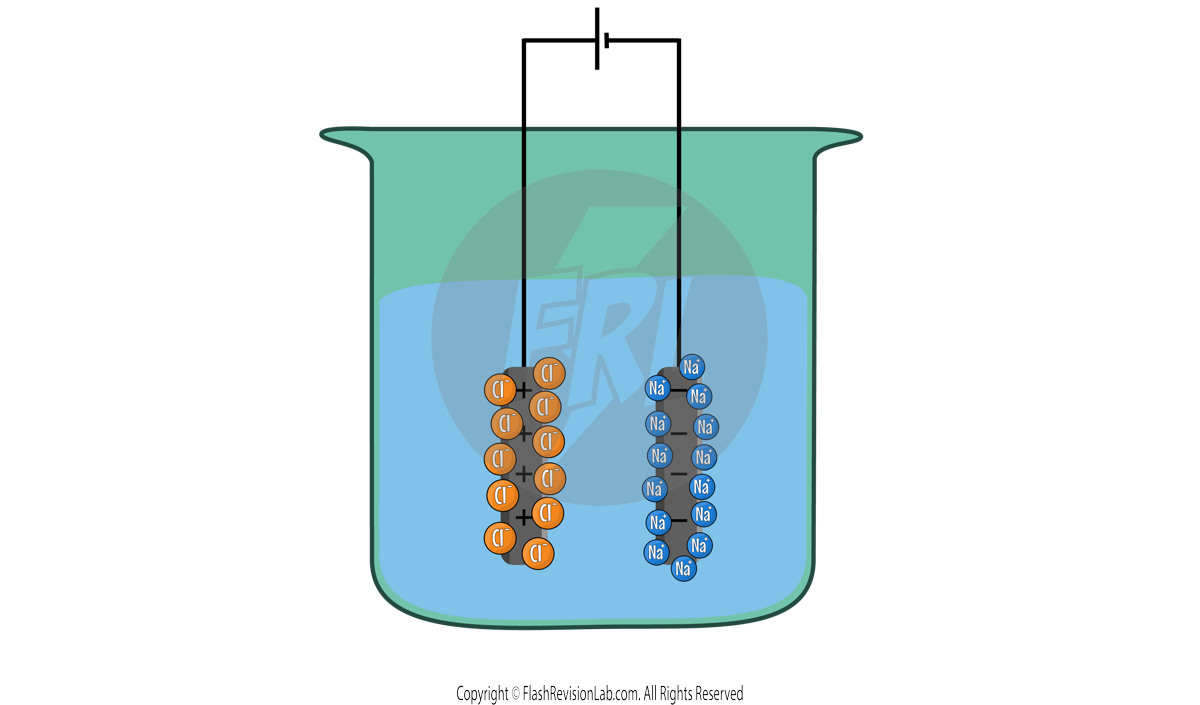
When they reach the anodes, both sets of ions TRANSFER ELECTRONS and turn into ATOMS of ELEMENTS.
Na+ IONS turn into Na ATOMS, while Cl- ions turn into Cl ATOMS. To represent these processes, you can write their HALF EQUATIONS.
HALF EQUATIONS
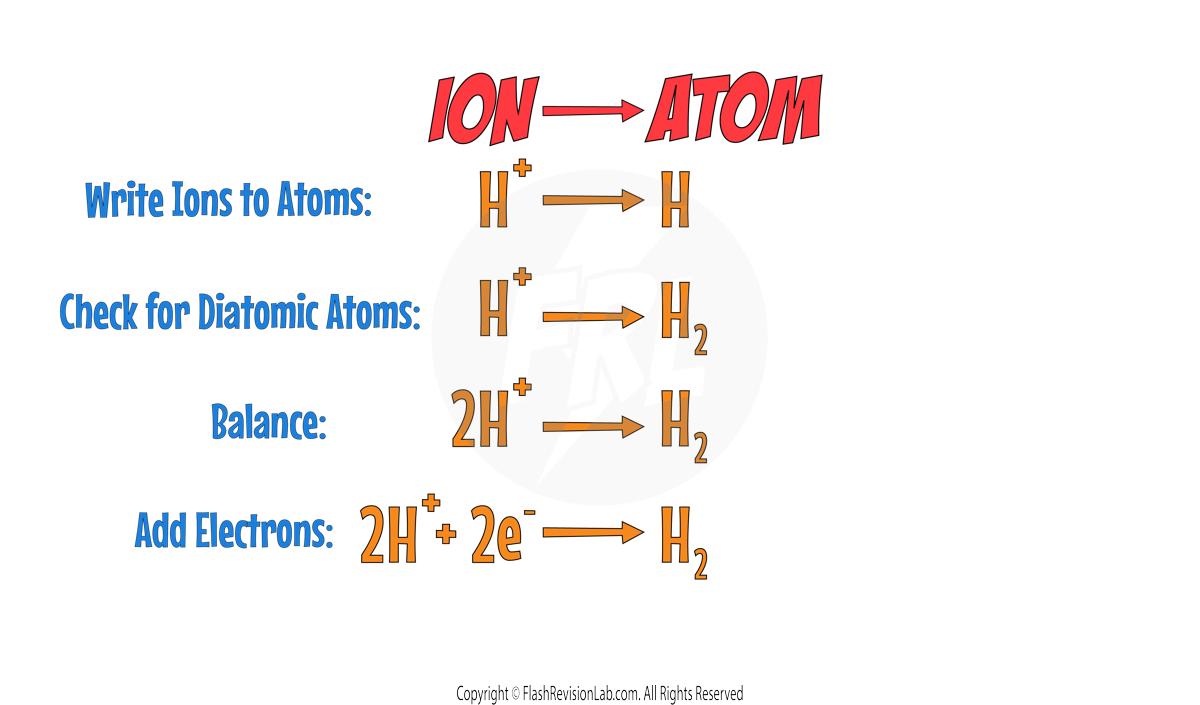
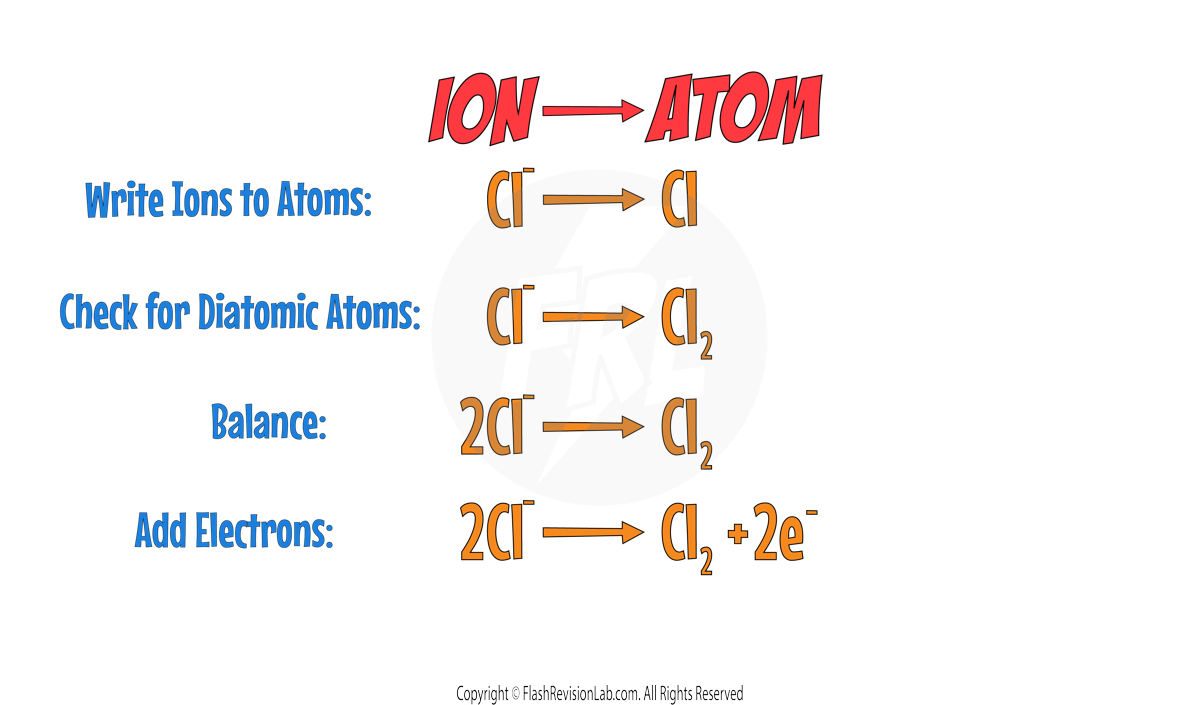
To write ELECTROLYSIS HALF EQUATIONS, you can follow these steps:
Step 1:
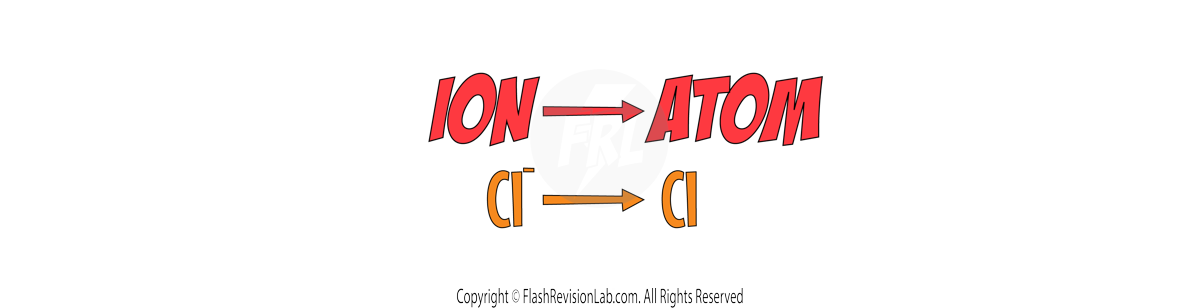
Write the ION of the element on the LEFT, and the ATOM of the element on the RIGHT.
Step 2:
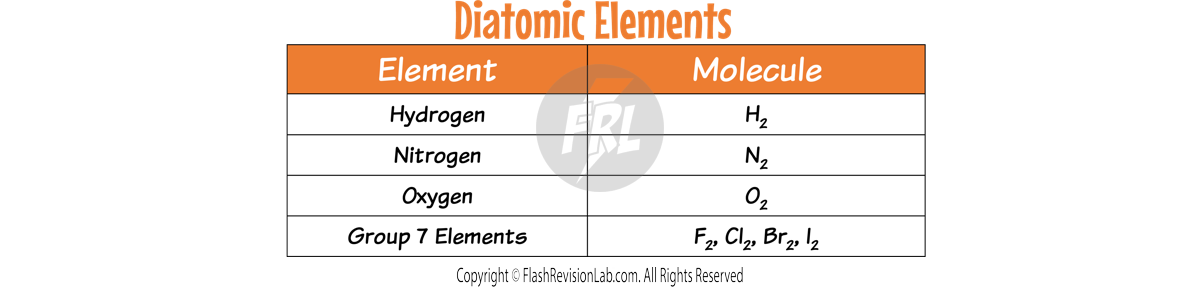
Check if any of the elements are DIATOMIC (exist as two atoms). Here is a list of ALL the DIATOMIC elements you need to know.
Chlorine is part of the list so it needs to be changed to Cl2.
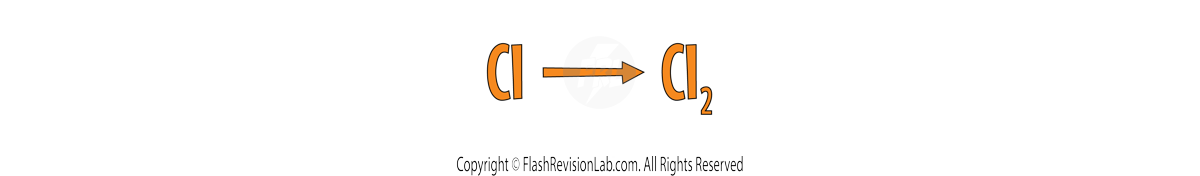
Step 3:
BALANCE the equation to make sure you have the same numbers on the left and right.
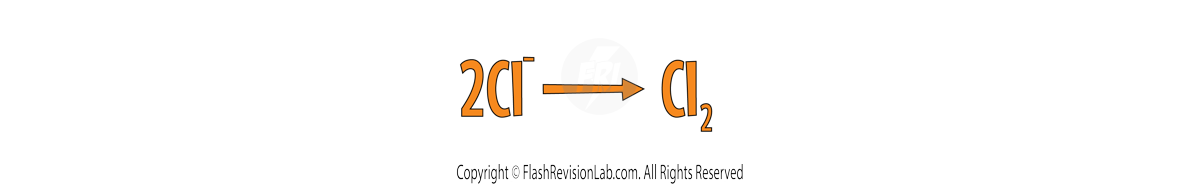
Step 4:
Add ELECTRONS to make the charges in the equation balanced.
- For ANODES, electrons go on the RIGHT.
- For CATHODES, electrons go on the LEFT.
In this example, each chlorine atom LOSES an electron so there are TWO electrons on the right. This gives the final ANODE half equation as:
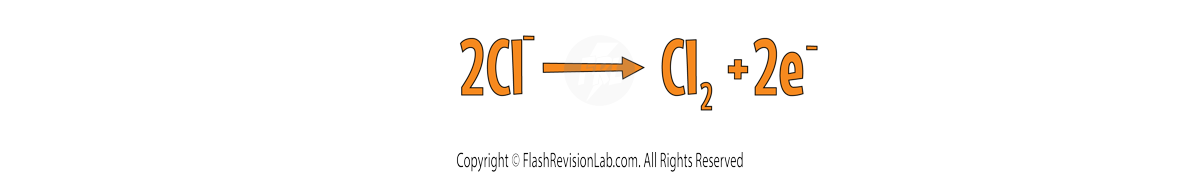
We can do the same for the CATHODE to find the half equation for Sodium.
Metal Extraction using Electrolysis
- Metals MORE reactive than CARBON can NOT be extracted by REDUCTION (See notes before).
- These metals need ELECTROLYSIS for extraction.
- A large amount of energy is required as the metal ore needs to be MELTED to become MOLTEN and ELECTRICITY is needed for the electrolysis. This makes the process EXPENSIVE.
EXTRACTION OF ALUMINIUM
- Aluminium is found as ALUMINIUM OXIDE ORE on Earth, and can be extracted using electrolysis.
- Before the ALUMINIUM OXIDE is MELTED, it is mixed with CRYOLITE, which LOWERS its MELTING POINT. This is useful as it means, LESS ENERGY is needed to melt it, making the process CHEAPER.
The set up for Aluminium extraction is slightly different to the normal classroom set up:
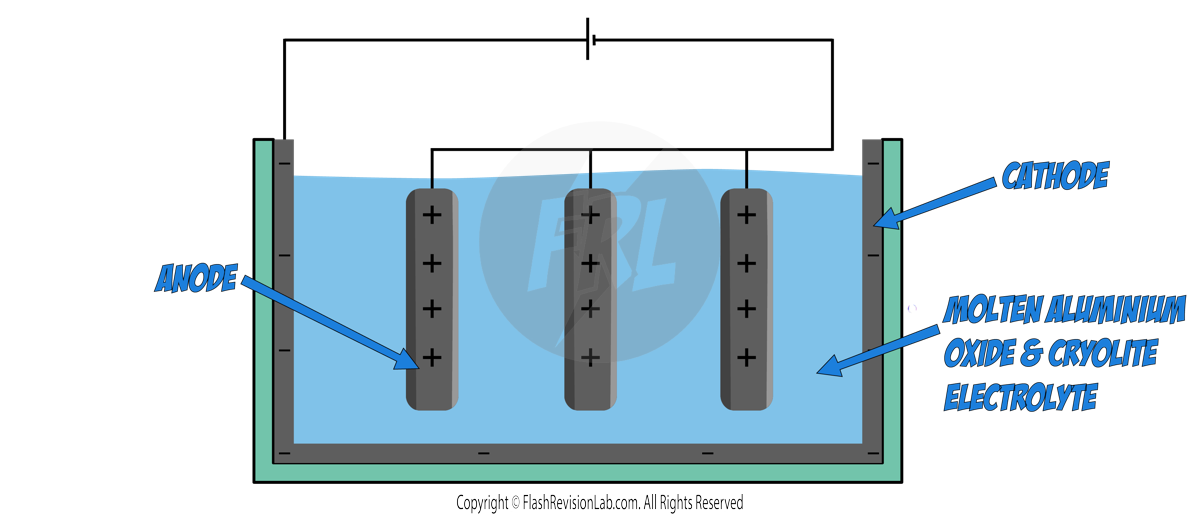
There is usually more than one anode submerged in the electrolyte, and the cathode lines the INSIDE of the container. Both electrodes are made of GRAPHITE.
The ions present in the liquid include POSITIVE ALUMINIUM IONS (Al3+) and NEGATIVE OXYGEN IONS (O2-).
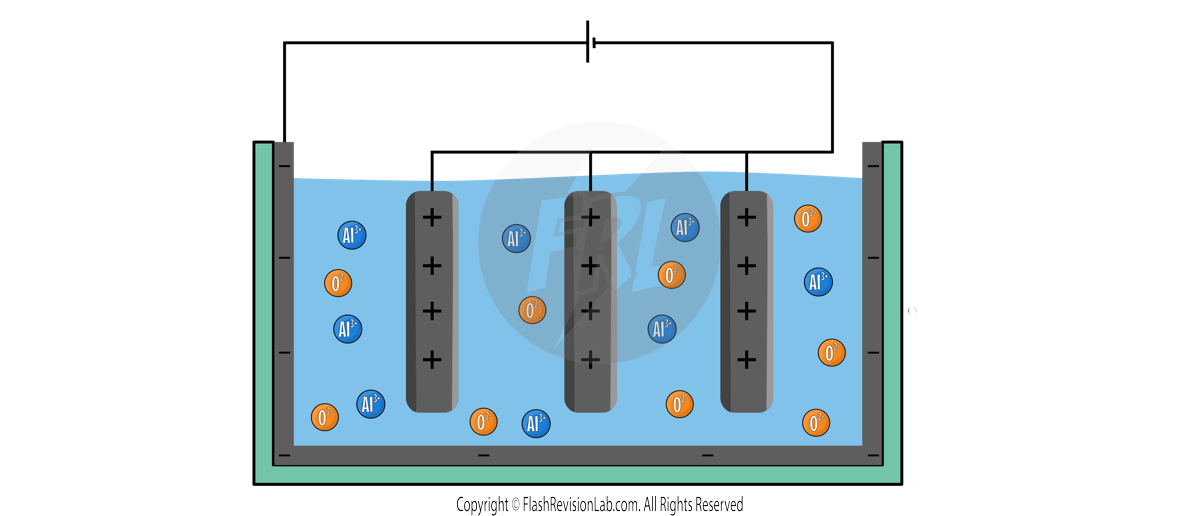
When current flows through the set up, the ALUMINIUM ions are attracted to the CATHODE, and the OXYGEN ions are attracted to the ANODE.
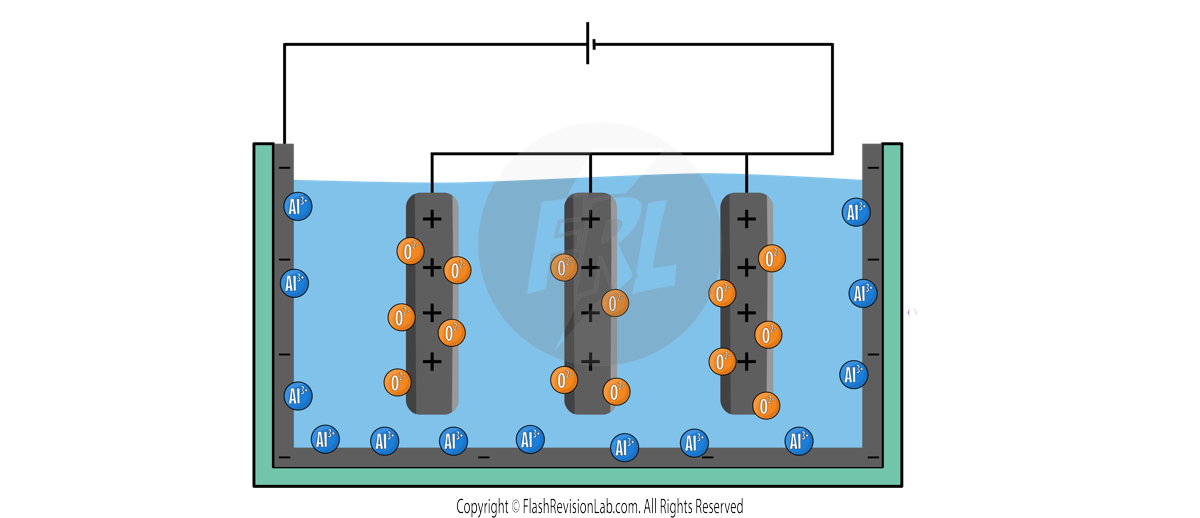
To find the HALF EQUATION for this process, you can follow the normal steps:
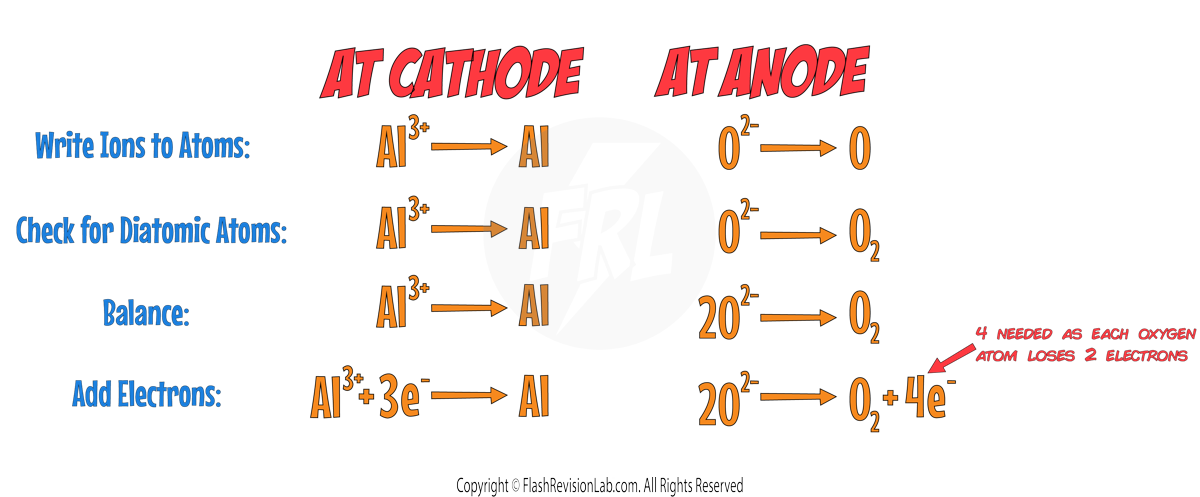
The final products in this electrolysis are ALUMINIUM metal and OXYGEN gas.
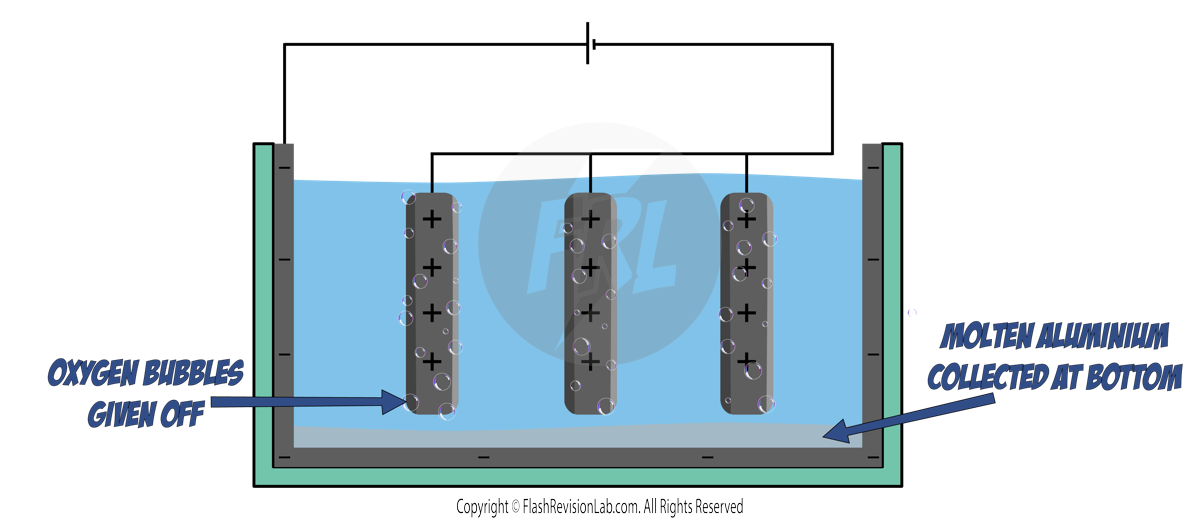
The Aluminium is produced as a MOLTEN LIQUID which flows to the bottom of the container due to the HIGH TEMPERATURE.
The Oxygen is produced as BUBBLES which float to the top of the liquid.
During this process the GRAPHITE ANODES need to be REPLACED REGULARLY. This is because they WEAR AWAY as the OXYGEN formed at the ANODES react with the CARBON in the graphite to form CARBON DIOXIDE.
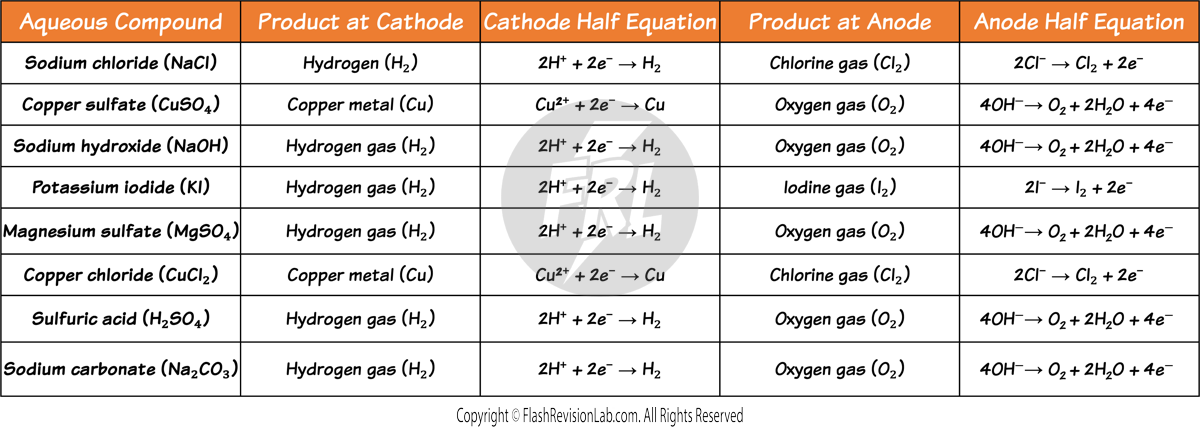
Electrolysis of Aqueous Solutions
- Ionic compounds in the AQUEOUS state conduct electricity because their IONS are FREE TO MOVE.
- Unlike MOLTEN electrolytes, the products formed in AQUEOUS electrolytes are not always the elements in the compound.
- This is because there are MORE THAN TWO DIFFERENT IONS present in an AQUEOUS SOLUTION.
IONS IN WATER
Just like acids do, water can partially IONISE to release HYDROGEN IONS (H+) and HYDROXIDE IONS (OH-) into the aqueous solution.
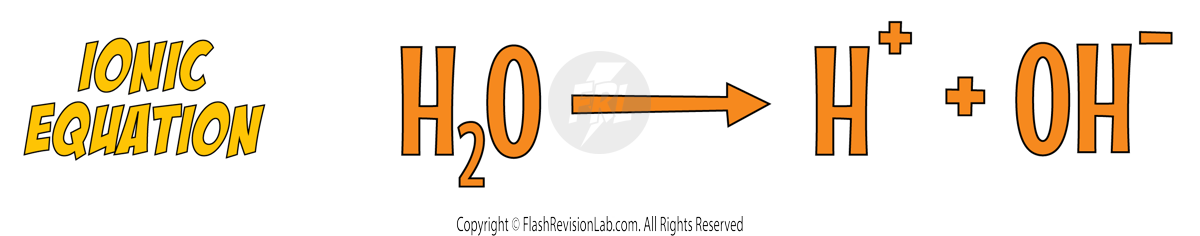
This means that the electrolyte can contain the ions from the ionic compound AND the ions from water ionising (H+ and OH-).
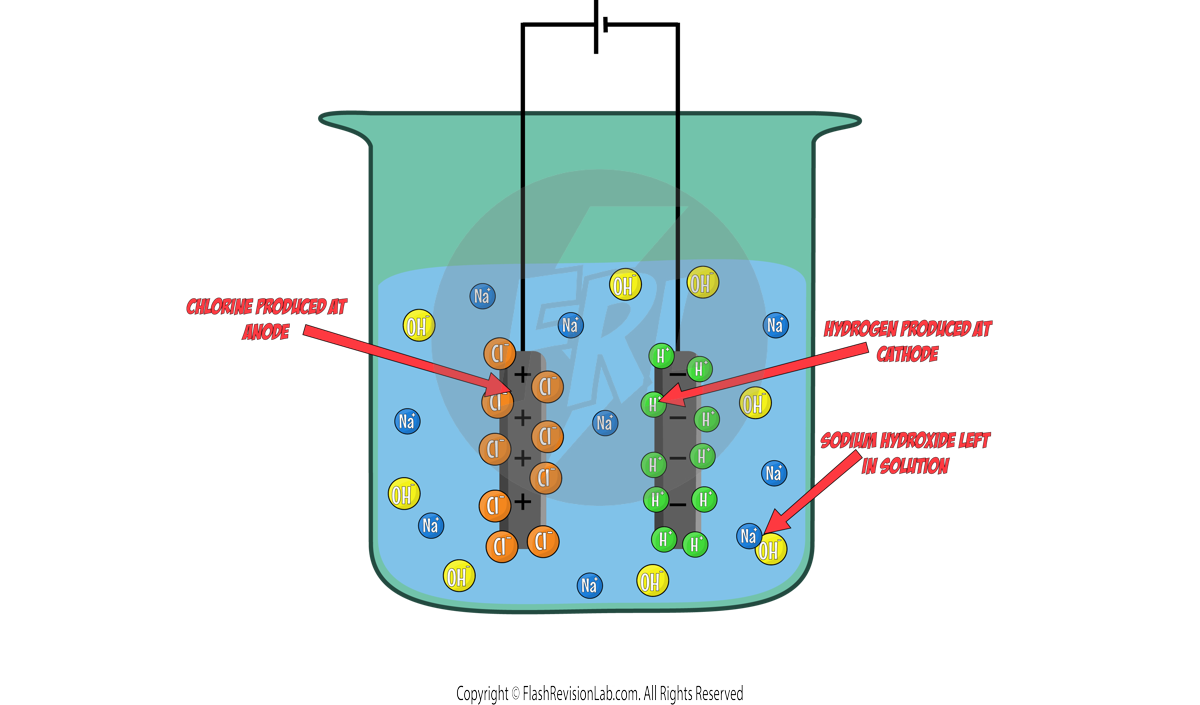
Let's use AQUEOUS SODIUM CHLORIDE as an example:
The ions present in this solution are:
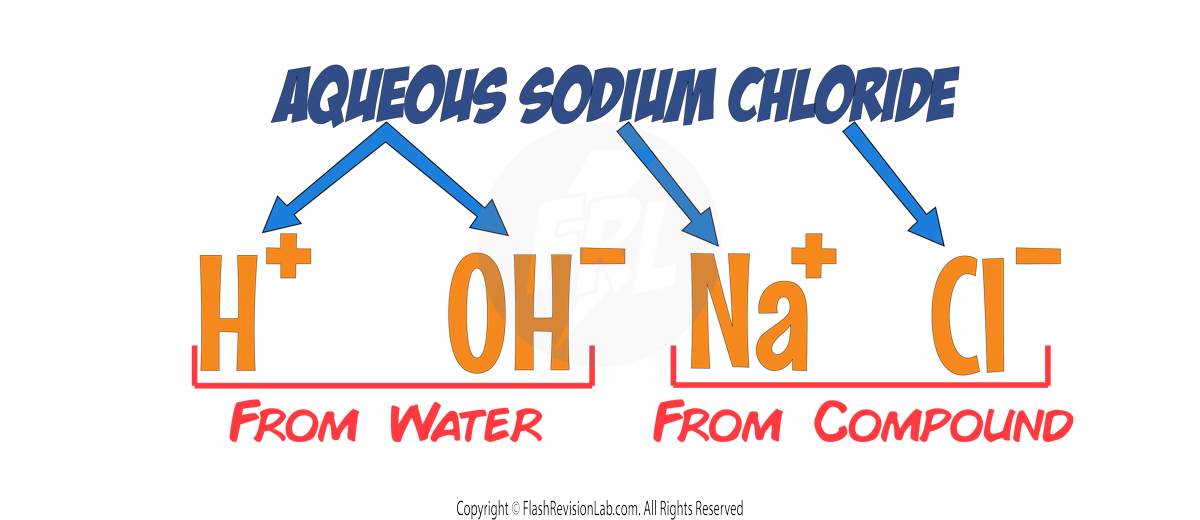
This means that all FOUR of these ions float around in the electrolyte.
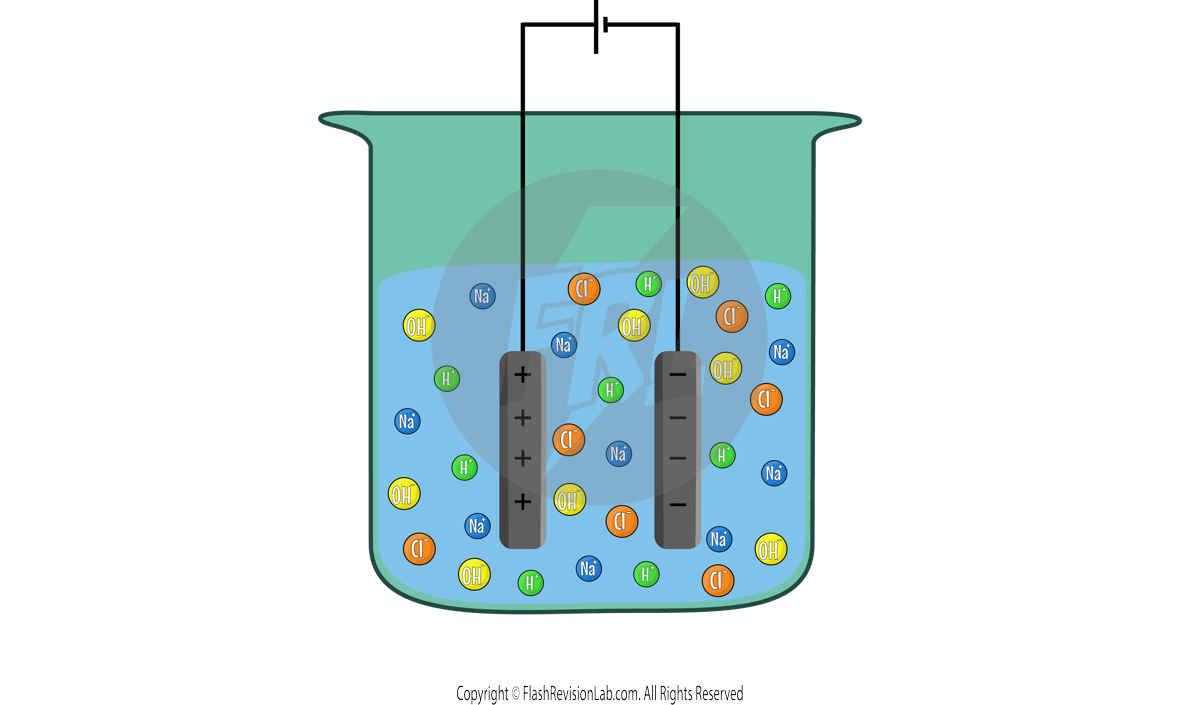
When the electricity flows through this, only ONE positive ion can go to the CATHODE and ONE negative ion can go to the ANODE.
DISCHARGING RULES FOR AQUEOUS ELECTROLYTES
To figure out which ions travel to the electrodes to get discharged in an aqueous solution, you need to follow TWO RULES:
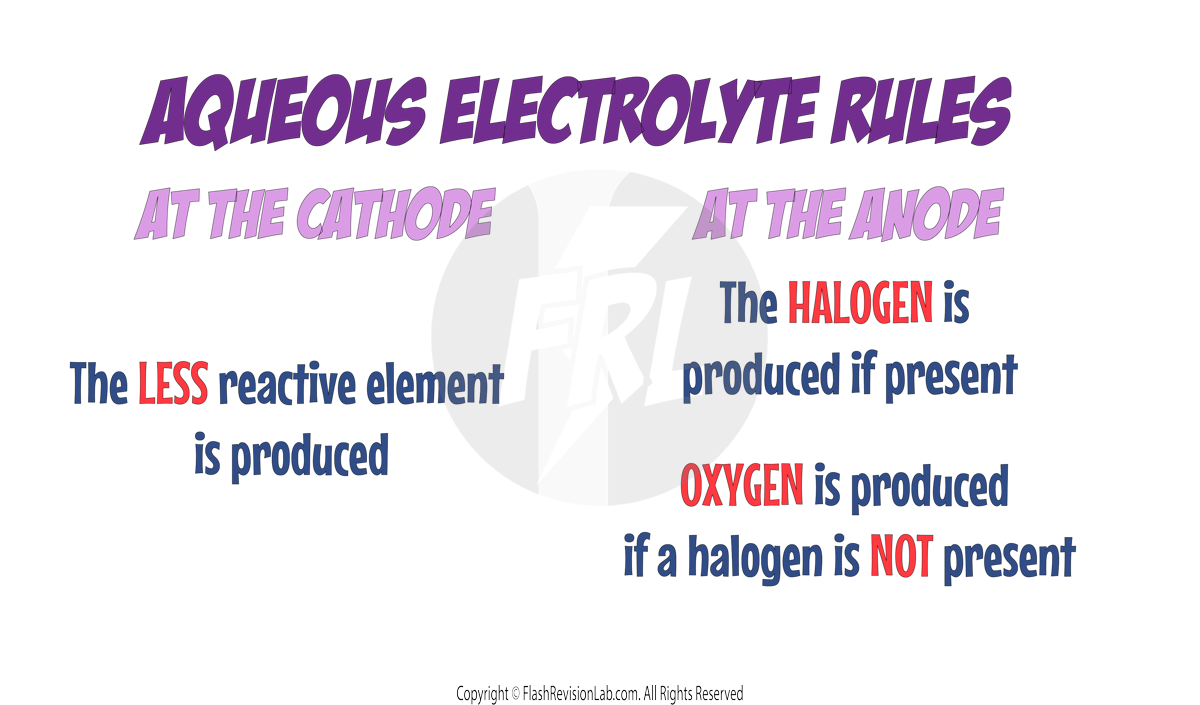
1. Rules for CATHODES
The LESS REACTIVE positive ions will travel to the CATHODE to be discharged, and its element will be formed.
2. Rules for ANODES
If a HALOGEN (Group 7 element) is present, the HALOGEN IONS will travel to the ANODE to be discharged to form its element.
If a halogen is NOT present, the HYDROXIDE IONS will travel to the ANODE to be discharged and to form OXYGEN GAS. If this occurs, the HALF EQUATION for this would be:
4OH−→ O2 + 2H2O + 4e−
You can use these rules in the Sodium Chloride example:
Cathode:
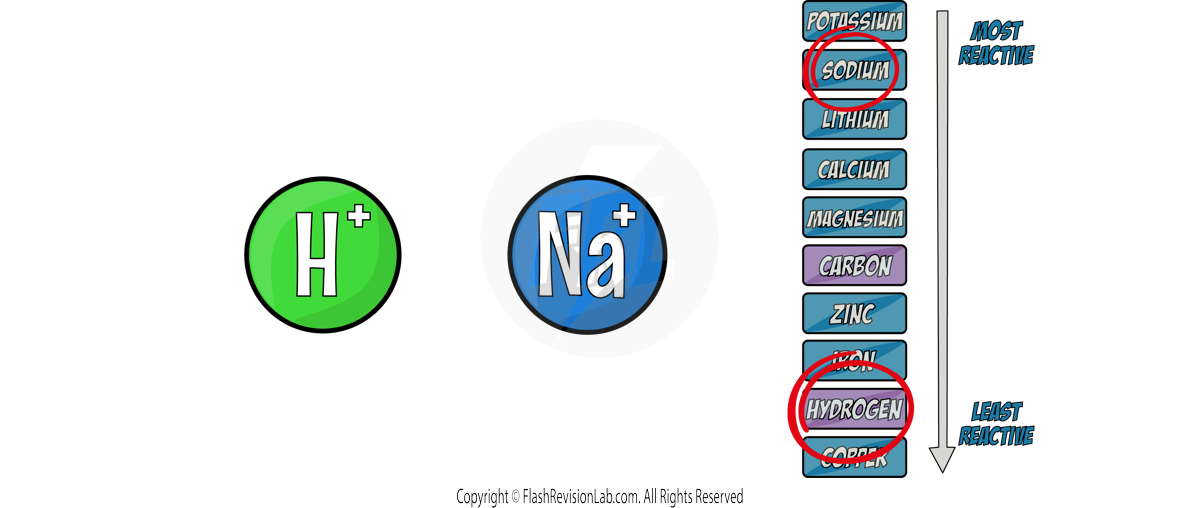
Out of the two positive ions, HYDROGEN is LESS reactive so it will go to the cathode. The HALF EQUATION for this can be worked out:
This means HYDROGEN gas will be given off at the cathode.
Anode:
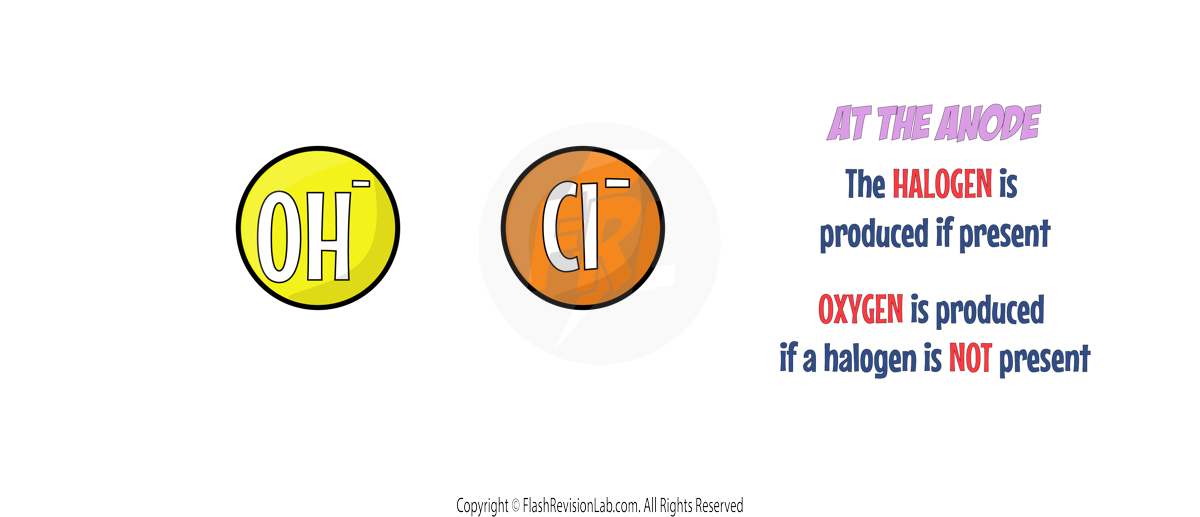
A halogen IS present therefore the CHLORINE will go to the anode. The HALF EQUATION will be:
This means CHLORINE gas will be given off at the anode.
Here are a few more examples of the half equations for different AQUEOUS ELECTROLYTES:
In the examples where METALS are formed in electrolysis with AQUEOUS ELECTROLYTES, you would observe the metal PLATING the surface of the electrode.
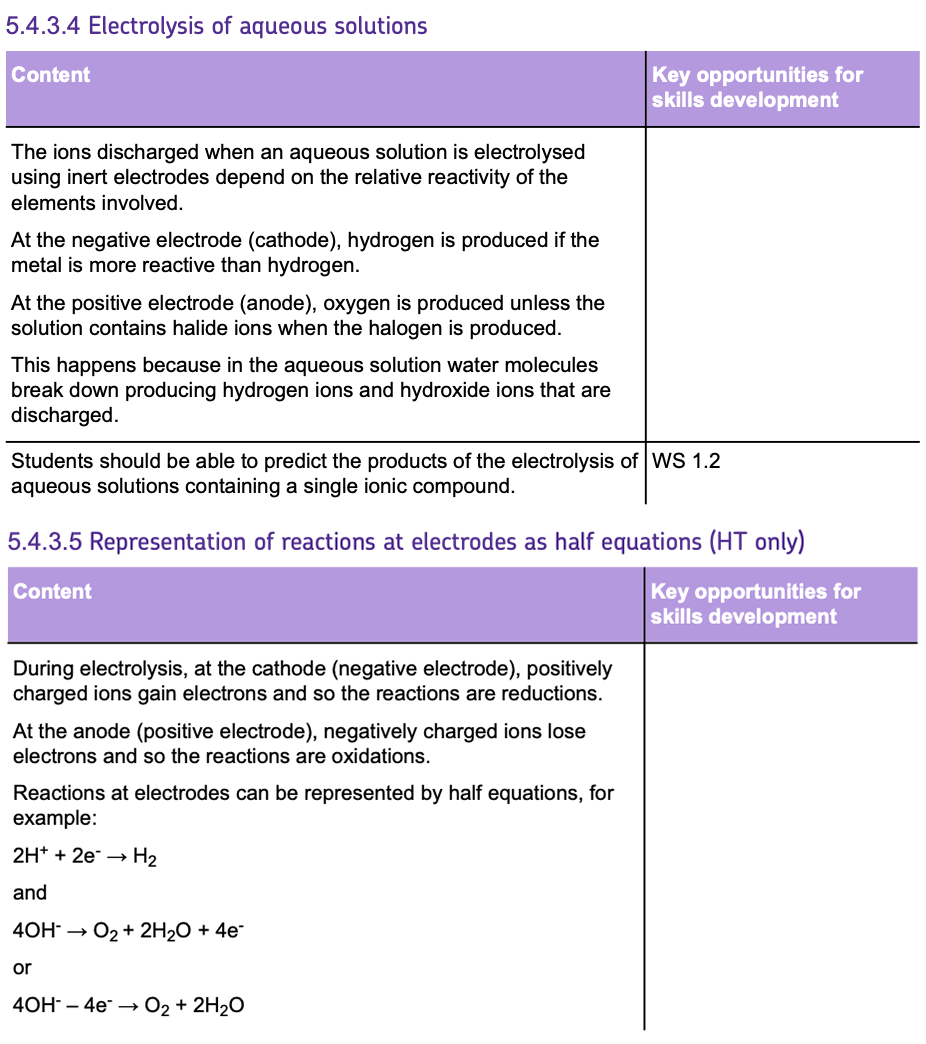
Required Practical: Electrolysis
This experiment is used to understand and observe the reactions occurring during the ELECTROLYSIS of various aqueous solutions using INERT ELECTRODES.
Equipment and Materials:
- TEST TUBES OR MEASURING CYLINDERS: To collect and measure gases produced at the electrodes.
- ELECTROLYTE SOLUTIONS: Aqueous solutions that will undergo electrolysis.
- BEAKER: To hold the electrolyte during electrolysis.
- GRAPHITE ELECTRODES: Inert electrodes that conduct electricity without reacting.
- POWER SUPPLY: To provide a controlled electric current.
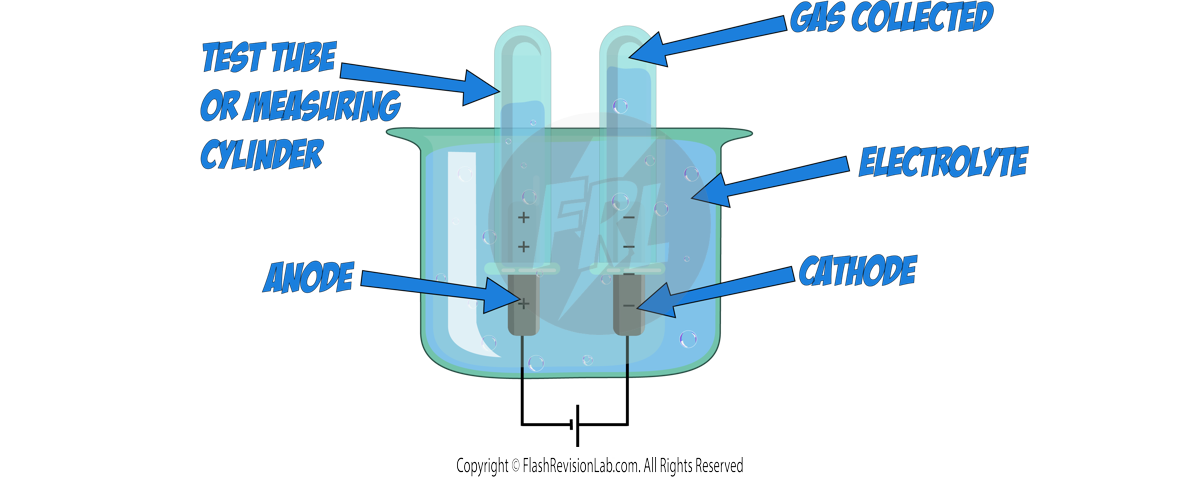
Method:
- Choose an ELECTROLYTE.
- Pour out a sample of the electrolyte into the beaker.
- Immerse two INERT electrodes into the solution and place an INVERTED TEST TUBE over each electrode.
- Pass CURRENT through the electrolyte and collect the gases in the inverted test tubes or measuring cylinders.
- Use CHEMICAL TESTS to identify the gases produced.
TEST FOR GASES
1. Chlorine:
Add DAMP BLUE LITMUS PAPER to the gas.
If the litmus paper BLEACHES WHITE, Chlorine is present.
2. Oxygen:
Add a GLOWING SPLINT to the gas.
If the glowing splint RELIGHTS, Oxygen is present.
3. Hydrogen
Add a LIT SPLINT to the gas.
If a SQUEAKY POP is observed, Hydrogen gas is present.

Exothermic and Endothermic Reactions
- Chemicals store energy in different amounts, so when chemical reactions occur, there is always a TRANSFER of energy when REACTANTS turn into PRODUCTS.
- This can be where reactants either ABSORB energy from the surroundings or RELEASE it.
- Energy can NOT be CREATED or DESTROYED, so the TOTAL amount of ENERGY in the UNIVERSE always stays the same BEFORE and AFTER a chemical reaction.
EXOTHERMIC REACTIONS:
- These are reactions that RELEASE ENERGY to the surroundings.
- They show a RISE IN TEMPERATURE.
- Common examples include COMBUSTION (like burning fuels), NEUTRALISATION reactions, and many OXIDATION REACTIONS such as metals reacting with acids.
Practical uses of Exothermic Reactions:
- HAND WARMERS: Utilise exothermic oxidation of iron.
- SELF-HEATING CANS: Depend on exothermic reactions for heating beverages.
ENDOTHERMIC REACTIONS:
- These reactions ABSORB ENERGY from the surroundings.
- They show a FALL IN TEMPERATURE.
- They are less common but can be found in processes like the reaction between CITRIC ACID and SODIUM HYDROGENCARBONATE, or THERMAL DECOMPOSITION.
Practical uses of Exothermic Reactions:
- SPORTS INJURY PACKS: The chemical reaction in these packs absorbs heat, causing them to cool without freezing.
Required Practical: Investigating Energy Changes
- ENERGY TRANSFER in chemical reactions can be measured by monitoring TEMPERATURE CHANGES.
- The LARGER the temperature change, the LARGER the energy transferred.
- You can use this to investigate how the amount of reactants in a reaction affects the amount of energy transferred.
- Variables like REACTANT MASS and CONCENTRATION can be investigated.
- You can apply the method to measure the energy change in NEUTRALISATION REACTIONS, reactions of METALS with ACIDS, DISPLACEMENT reactions, and reactions between CARBONATES and ACIDS.
Set Up:
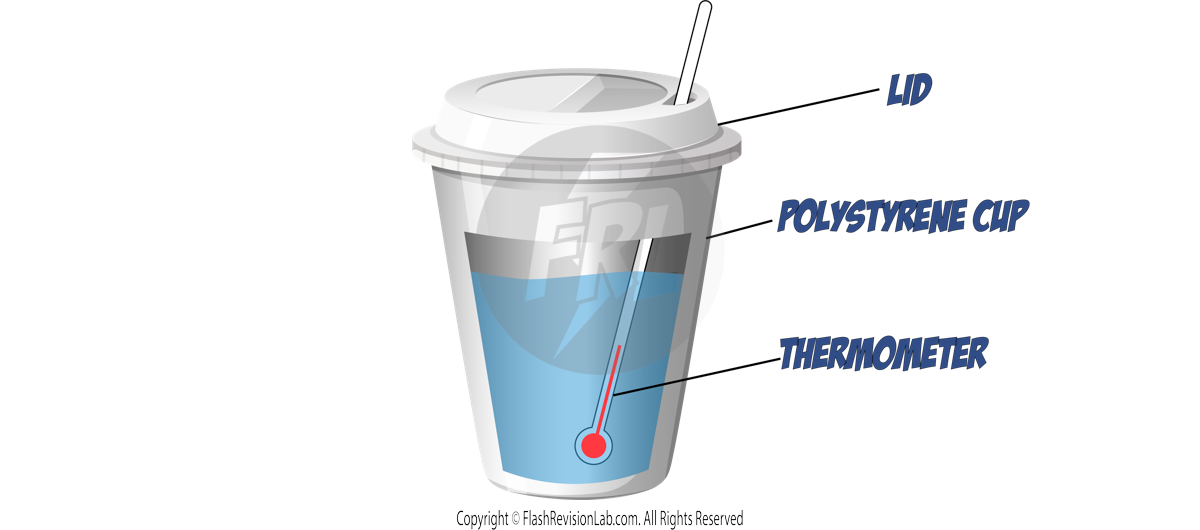
- Insulation: Use a POLYSTYRENE CUP with a lid for insulation to minimise energy loss to the surroundings.
Method for a Neutralisation Reaction:
- Prepare separate solutions of Hydrochloric acid (HCl) and Sodium Hydroxide (NaOH) with known concentrations.
- Measure the INITIAL TEMPERATURE of the solutions.
- Mix the reactants in the insulated cup and immediately cover with a lid to prevent heat loss.
- Stir the solution.
- Measure the temperature at regular intervals (e.g. every 30 seconds) and record the HIGHEST temperature reached.
- Calculate the temperature difference between the INITIAL and HIGHEST temperature readings.
- Repeat this process for DIFFERENT CONCENTRATIONS of acid.
Method for a Reaction between an Acid and a Metal:
- Add a solution of Hydrochloric acid (HCl) to the insulated cup.
- Measure the INITIAL TEMPERATURE of the solutions.
- Add a KNOWN MASS of Magnesium and immediately cover with a lid to prevent heat loss.
- Stir the solution.
- Measure the temperature at regular intervals (e.g. every 30 seconds) and record the HIGHEST temperature reached.
- Calculate the temperature difference between the INITIAL and HIGHEST temperature readings.
- Repeat this process for DIFFERENT MASSES of Magnesium.

Reaction Profiles
REACTION PROFILES are graphs that show how ENERGY CHANGES throughout a reaction.
The Y-AXIS represents the ENERGY and the X-AXIS represents the PROGRESS OF REACTION.
The difference in HEIGHT between the REACTANTS and PRODUCTS represents the OVERALL ENERGY CHANGE of the reaction.
The curved lined between the reactants and products represents how the energy changes as the reaction proceeds.
The difference in height between the REACTANTS and the PEAK of the graph represents the ACTIVATION ENERGY.
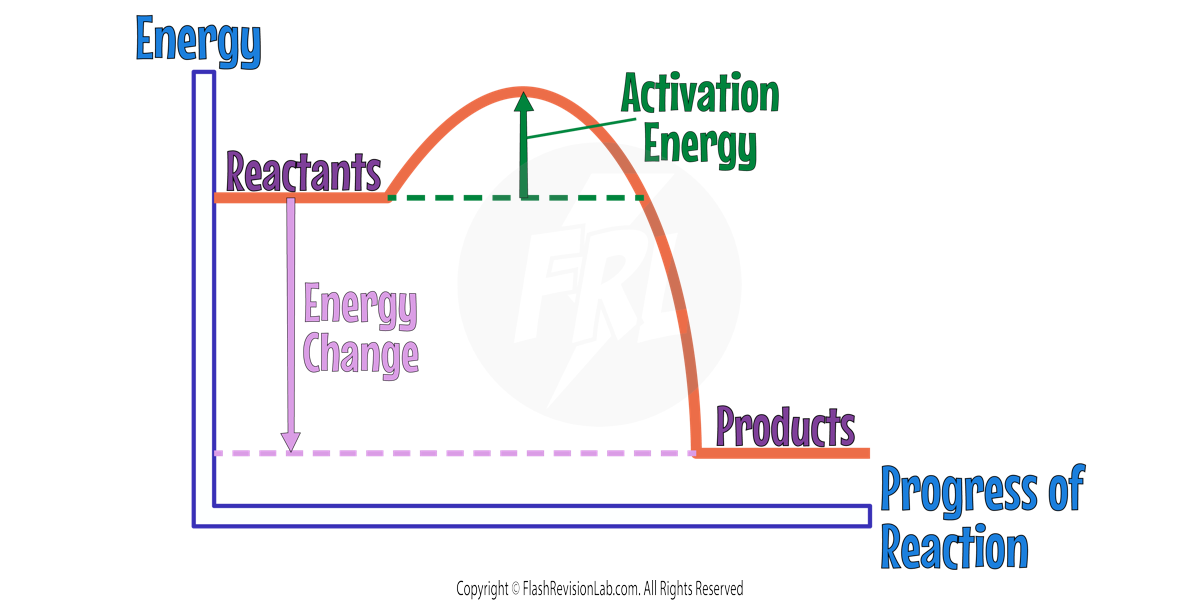
ACTIVATION ENERGY
Chemical reactions can occur only when reacting particles collide with each other and with SUFFICIENT ENERGY.
The ACTIVATION ENERGY represents the MINIMUM ENERGY needed for reactant particles to COLLIDE with each other, in order to REACT.
The BIGGER the activation energy peak, the greater the ACTIVATION ENERGY.
EXOTHERMIC AND ENDOTHERMIC REACTION PROFILES
Both exothermic and endothermic reactions have different reaction profiles.
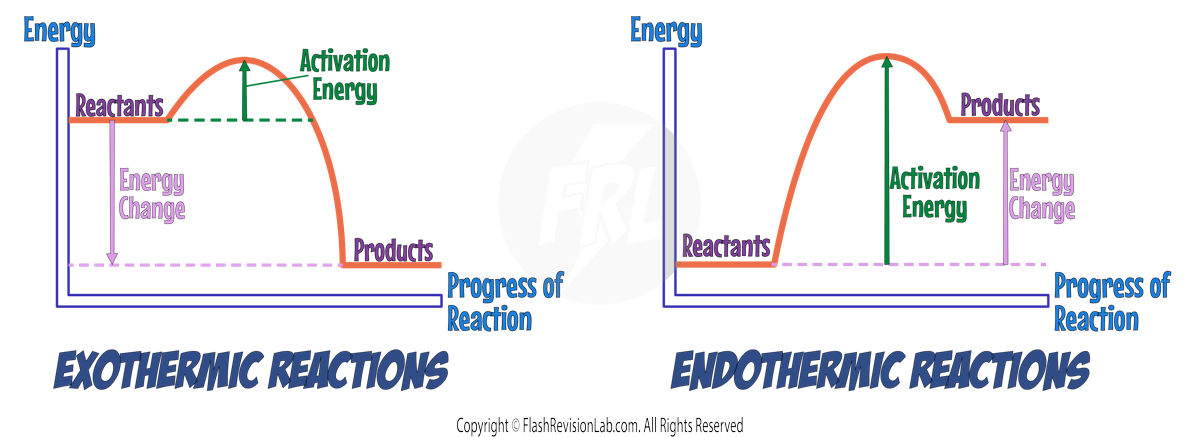
EXOTHERMIC REACTIONS:
- These RELEASE ENERGY, with products having a LOWER ENERGY than reactants.
- ENERGY RELEASED is shown as a DROP in energy.
ENDOTHERMIC REACTIONS:
- These absorb energy, resulting in products at a HIGHER ENERGY LEVEL than reactants.
- ENERGY ABSORBED is shown by a RISE in energy.
Energy Changes in Reactions
During any reaction there are TWO processes that occur:
1. Bond Breaking
2. Bond Forming
Let’s look at the reaction between Hydrogen and Chlorine as an example:
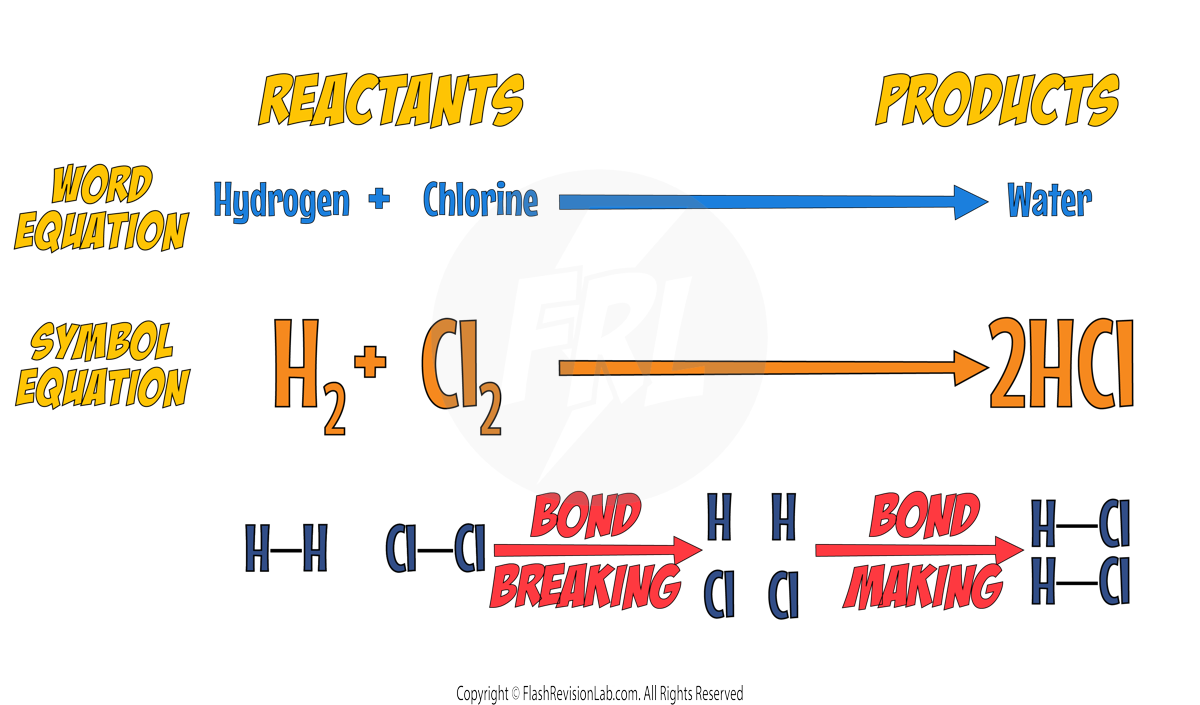
BOND BREAKING:
This is where all the bonds in the reactants are BROKEN.
This process TAKES IN ENERGY and is ENDOTHERMIC.
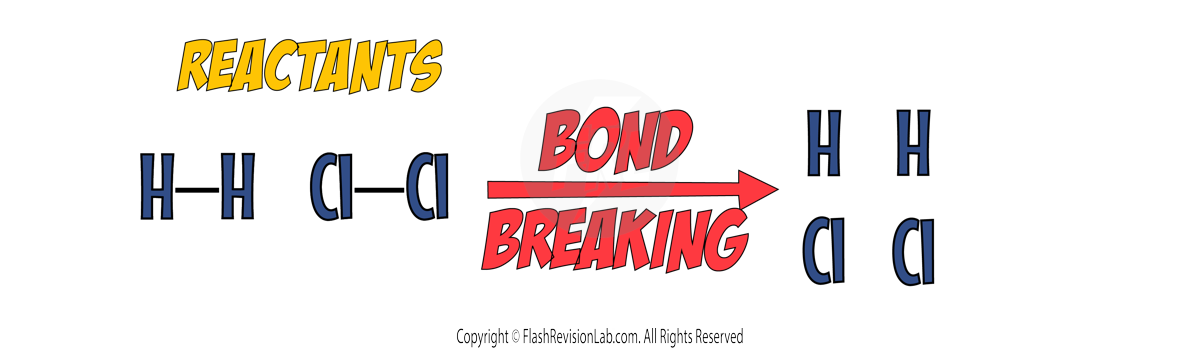
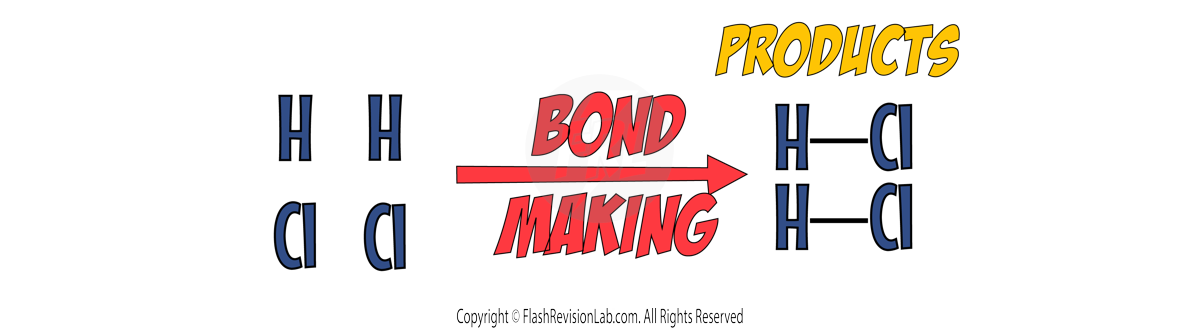
BOND FORMING:
The atoms rearrange themselves and new BONDS ARE FORMED to create the products.
This process RELEASES ENERGY, making it an EXOTHERMIC process.
EXOTHERMIC AND ENDOTHERMIC REACTIONS
The amount of energy RELEASED and TAKEN in from these two processes governs whether a reaction is EXOTHERMIC or ENDOTHERMIC:
- In an EXOTHERMIC reaction, more ENERGY is RELEASED during BOND FORMING than is TAKEN IN in BOND BREAKING.
-
In an ENDOTHERMIC reaction, more energy is TAKEN IN during BOND BREAKING than is RELEASED during BOND FORMING.
Bond Energy Calculations
Using these calculations, you can work out the ENERGY CHANGE in a reaction by using BOND ENERGY values.
BOND ENERGIES tell you the amount of energy needed to break a specific bond. These are measured in kJ/mol and are always given to you in exams.
To calculate the ENERGY CHANGE, you need to use the following equation:
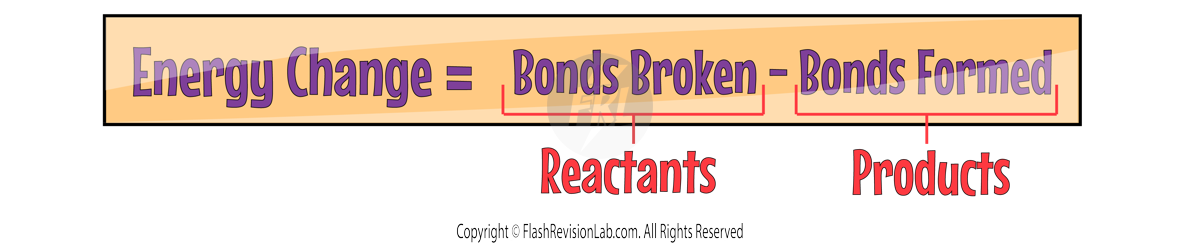
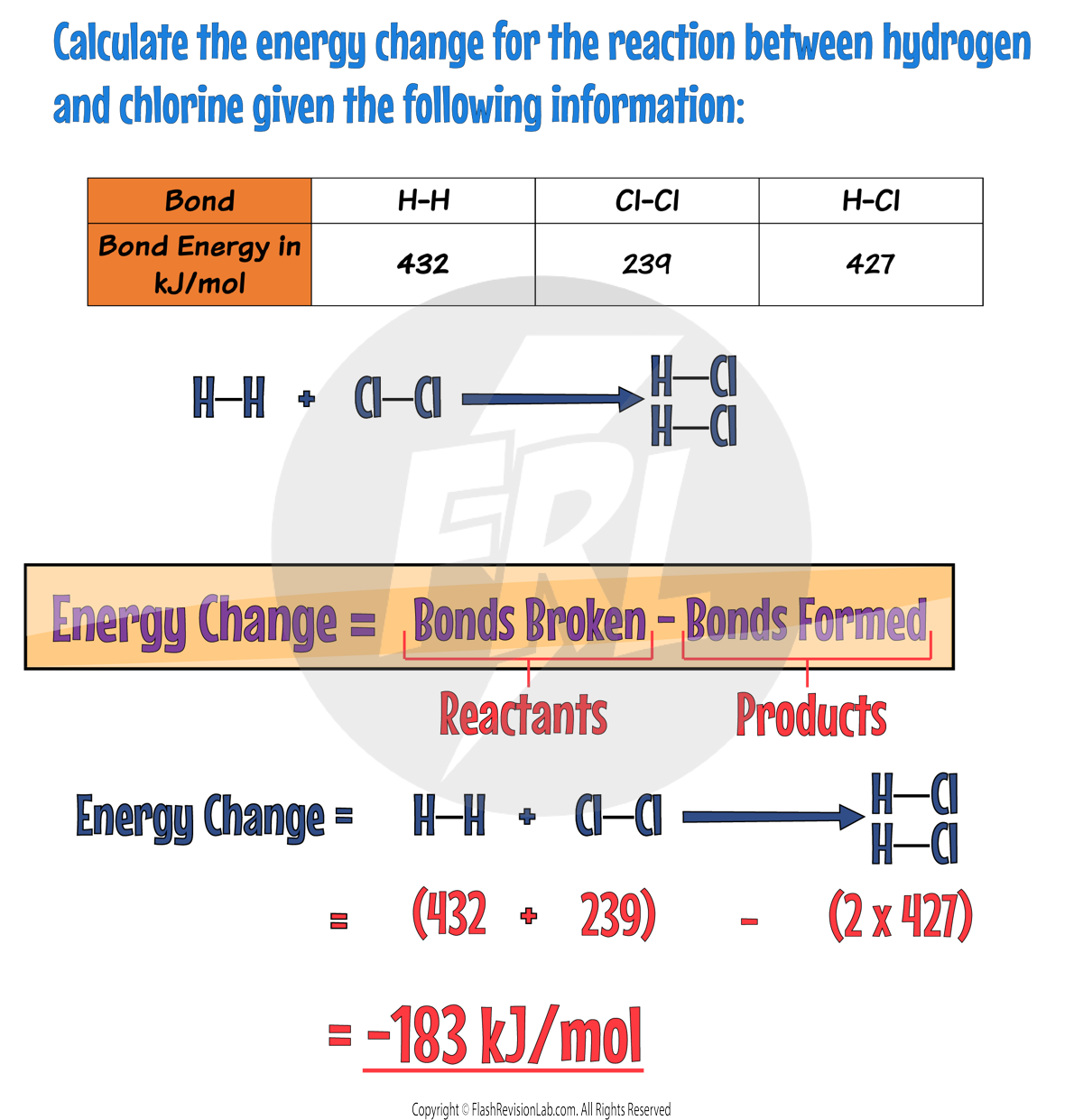
Let's try to answer the following question:
Calculating Rate of Reaction
The RATE of a reaction tells you how FAST the reaction occurs and can be determined by two factors:
- The amount of REACTANTS USED UP in a given time.
- The amount of PRODUCTS FORMED in a given time.
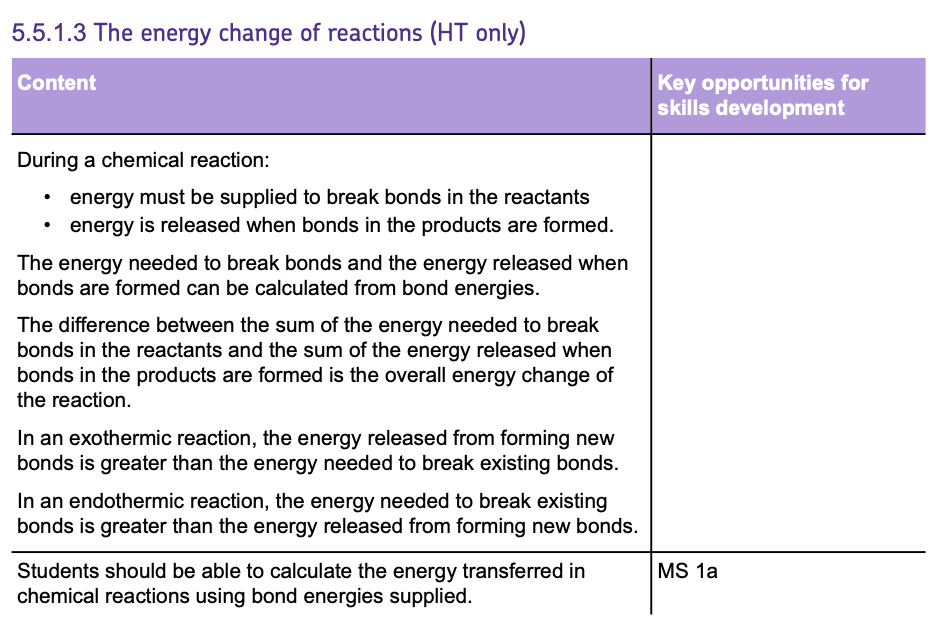
You can find the rate of a reaction by using the following equations:

The UNITS of rate of reaction are usually given in:
- GRAMS PER SECOND (g/s)
- CENTIMETRES CUBED PER SECOND (cm³/s)
- MOLES PER SECOND (mol/s)
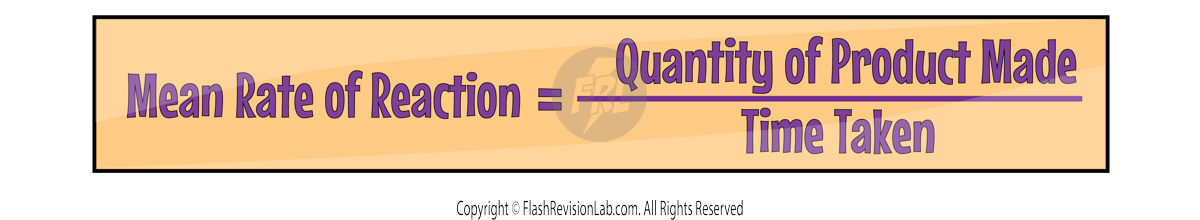
Here is an example of a calculation:
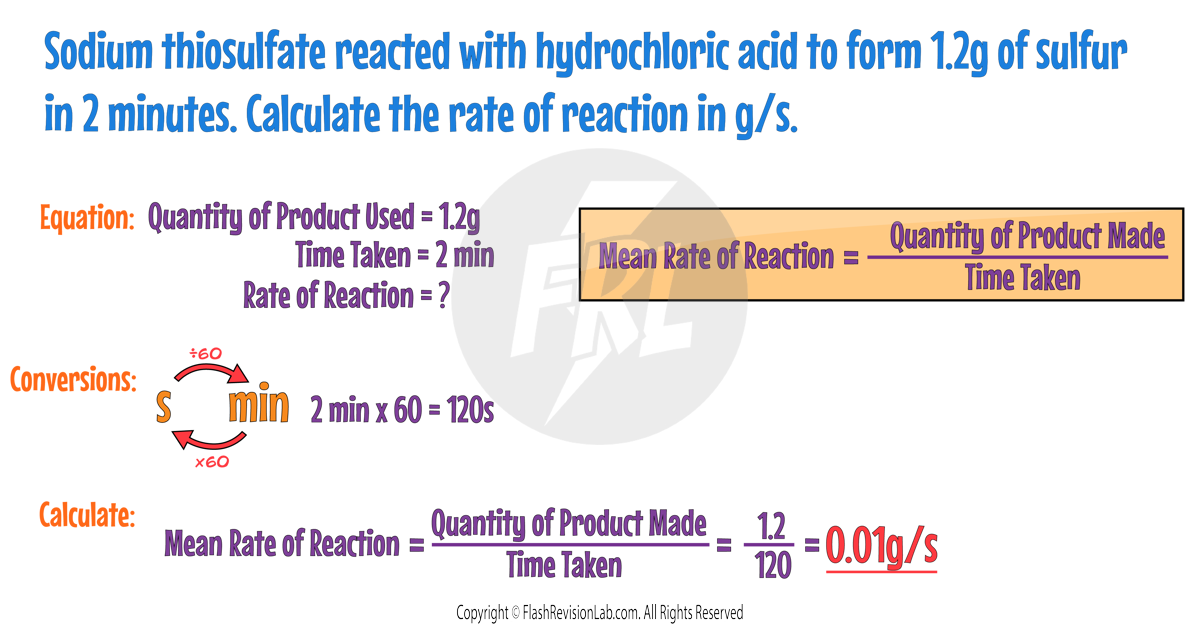
RATE GRAPHS
Graphs of "reactants used up" or "products formed" can show how the rate changes throughout a reaction. Generally these graphs would look like this:
The rate of reaction is represented by the GRADIENT (steepness) of the line.
The STEEPER the line, the FASTER the rate of reaction.
Both graphs are the STEEPEST at the start, showing that the rate is the FASTEST when the reaction first begins.
The GRADIENT DECREASES as the reaction goes on because the reactant particles get USED UP.
When the line becomes FLAT the reaction is COMPLETE.
GRADIENTS
You can work out the GRADIENT of a line by dividing the 'change in y' value by the 'change in x' value.
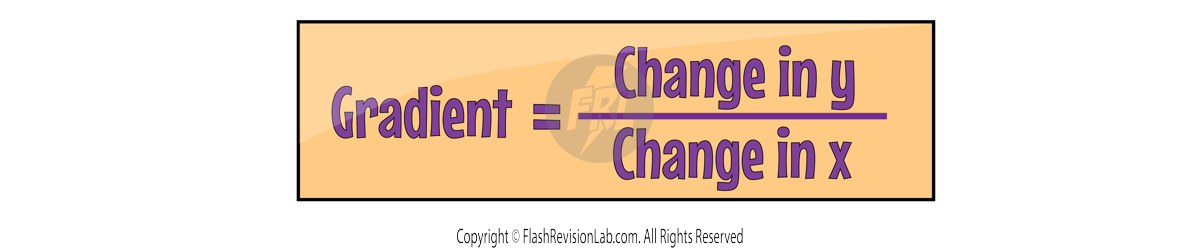
Straight Lines
If the line is STRAIGHT, you can pick TWO POINTS and draw a TRIANGLE from them. Then divide the CHANGE IN Y (vertical length) by the CHANGE IN X (horizontal length).
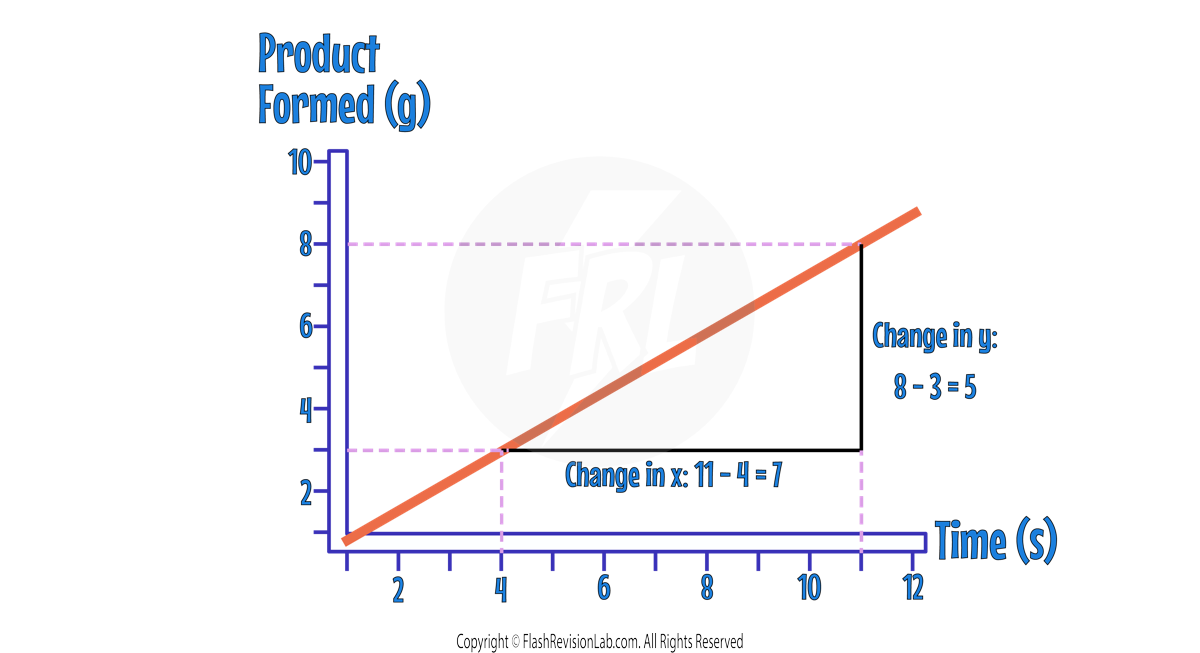
In this example, the rate would be calculated as 5/7 which gives a rate of 0.71 g/s.
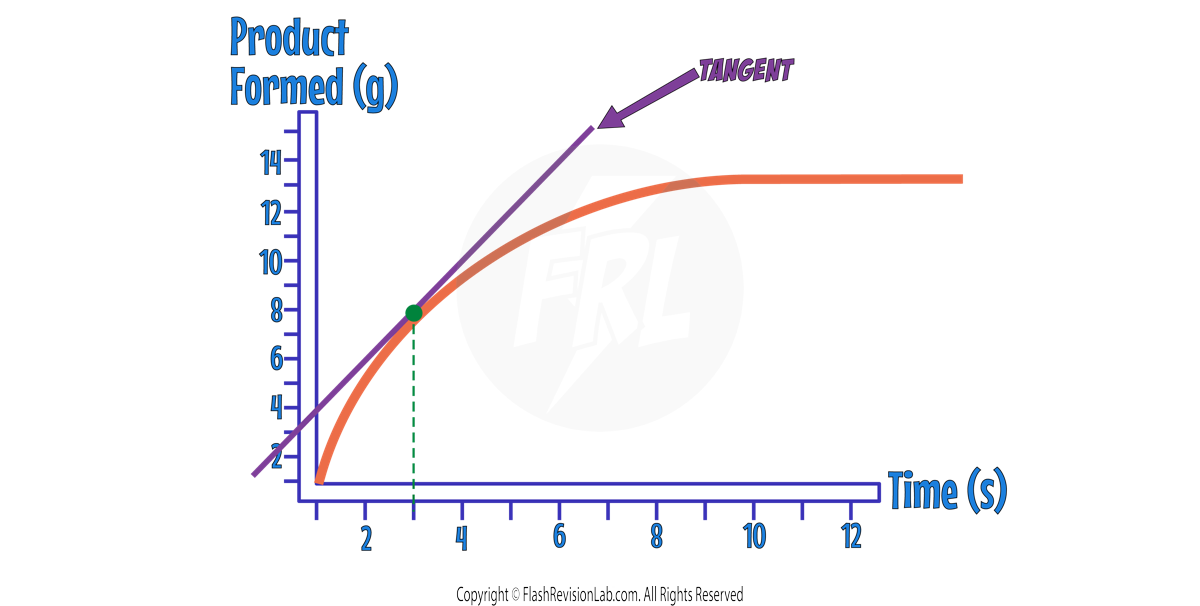
Curved Lines
A curved line is one where the GRADIENT CHANGES.
You can work out the rate at a particular time by drawing a TANGENT.
A TANGENT is a line which touches the curve at ONE POINT and has the SAME GRADIENT as that point.
For example, if you wanted to find the rate of reaction after 3s, you would draw your tangent so it touches the curve at 3s.
Now you can find the GRADIENT by drawing a triangle and finding the 'change in y' and the 'change in x'.
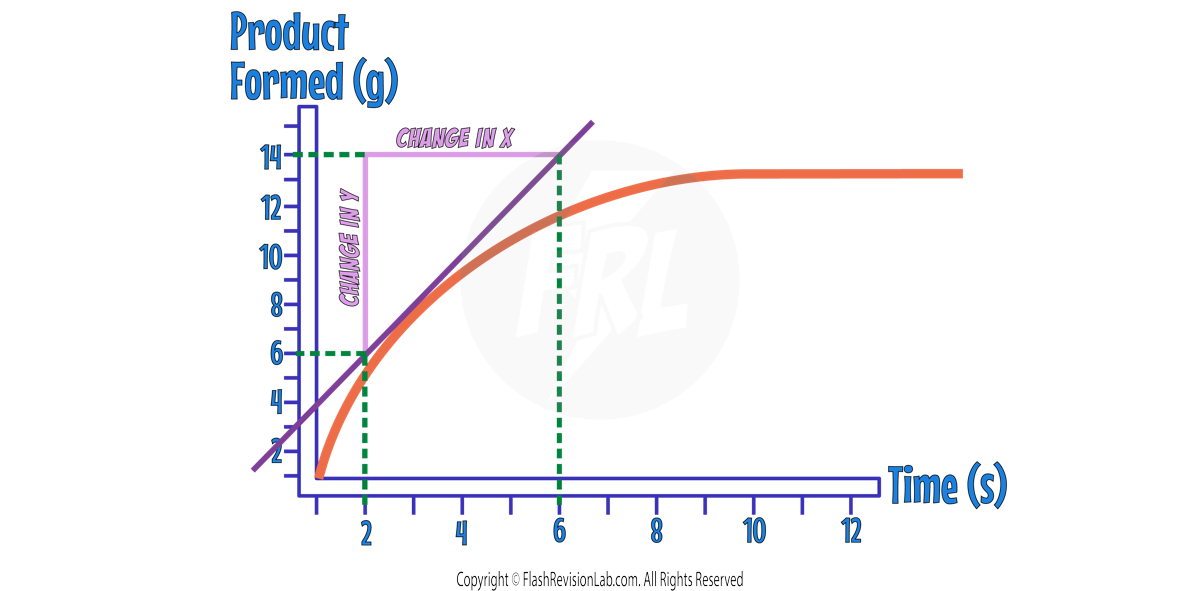
Change in y = 14 – 6 = 8
Change in x = 6 – 2 = 4
Gradient = 8/4 = 2 g/s
The rate of the reaction at 3s is 2g/s.

Required Practical: Rates of Reaction
Measuring TURBIDITY or COLOUR
Turbidity is a measure of how CLEAR a liquid is. Some solutions can react to form a SOLID PRECIPITATE. This can cause the liquid to turn CLOUDY, making it more difficult to see THROUGH the solution.
E.g. A reaction between SODIUM THIOSULFATE and HYDROCHLORIC ACID.
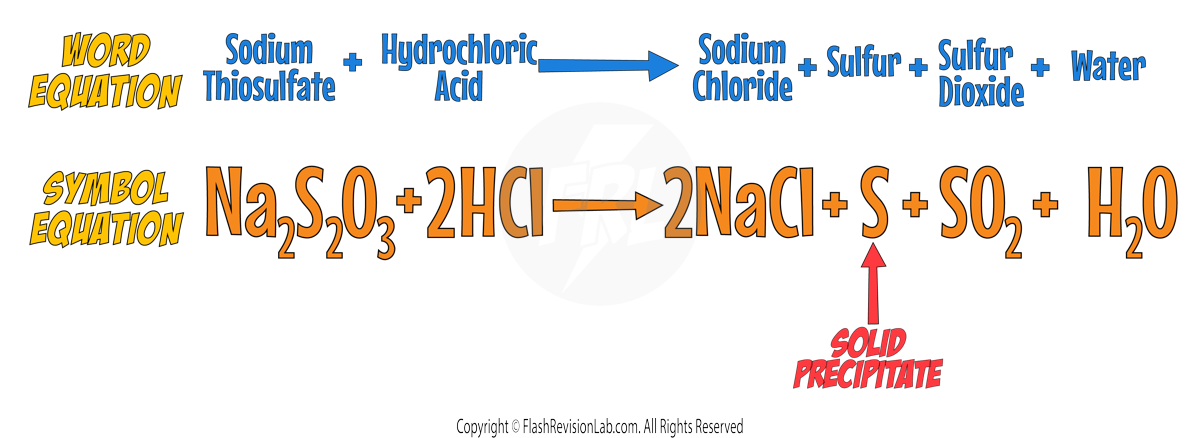
The Sulfur precipitate produced causes the solution to turn CLOUDY.
The FASTER the precipitate forms, the FASTER the RATE.
Equipment:
- SODIUM THIOSULFATE solution
- HYDROCHLORIC ACID
- CONICAL FLASK
- WHITE PAPER with BLACK CROSS on it
- STOPWATCH or timer
Method:
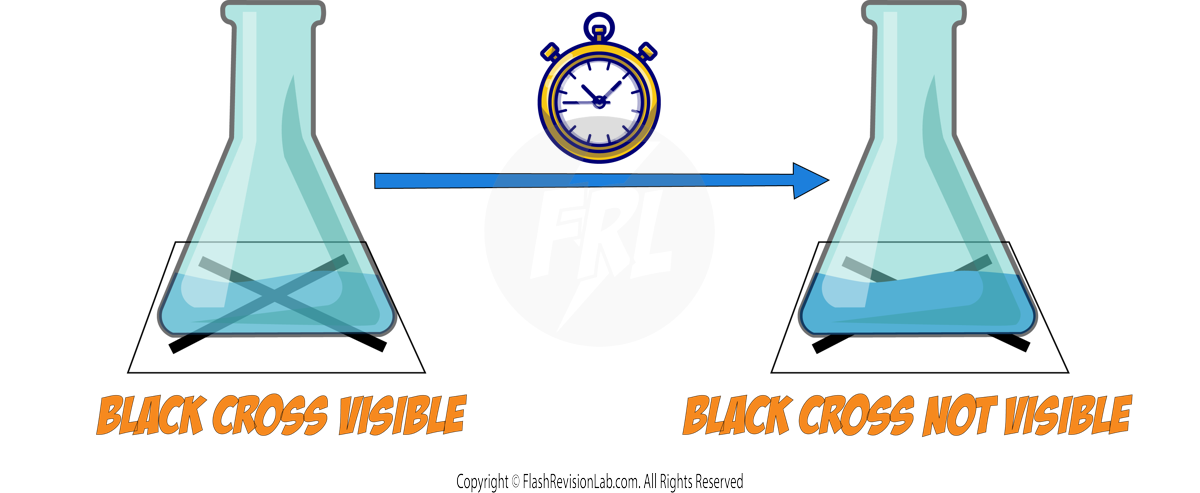
- Measure a specific volume of SODIUM THIOSULFATE solution into a CONICAL FLASK.
- Measure a specific volume of HYDROCHLORIC ACID into a measuring cylinder.
- Place a piece of white paper with a BLACK CROSS under the CONICAL FLASK.
- Pour the acid into the flask and start the STOPWATCH.
- As SOLID SULFUR forms, a CLOUDY PRECIPITATE is observed.
- Stop the stopwatch when the BLACK CROSS is no longer visible through the cloudy mixture.
- Repeat this method for different CONCENTRATIONS of SODIUM THIOSULFATE.
Results:
The SHORTER the time taken for the cross to disappear, the FASTER the rate of reaction.
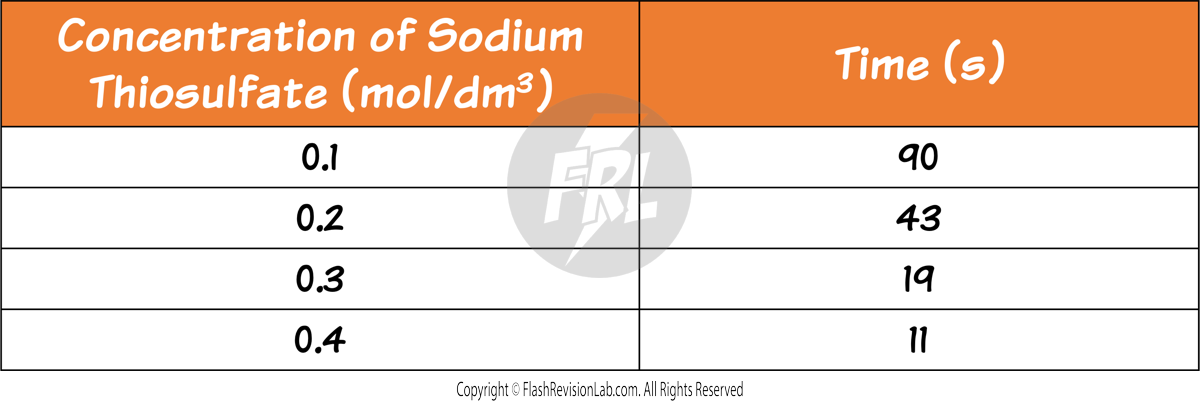
From these results you can see that the MORE CONCENTRATED the solution is, the FASTER the rate of reaction.
These experiments are not accurate as the results are SUBJECTIVE. The point at which the cross disappears would be judged differently by different people.
A similar method can be used for reactions that involve a COLOUR CHANGE. The person measuring the time judges the point at which the colour of the solution changes.
Measuring Gas Volume in Reactions
In reactions where a GAS is given off, the FASTER the gas is produced, the FASTER the RATE of reaction.
E.g. A reaction between MAGNESIUM and HYDROCHLORIC ACID.

You can use a GAS SYRINGE to monitor the volume of gas collected.

Equipment:
- MAGNESIUM RIBBON
- HYDROCHLORIC ACID
- CONICAL FLASK
- SAFETY GOGGLES
- GAS SYRINGE
- STOPWATCH
Method:
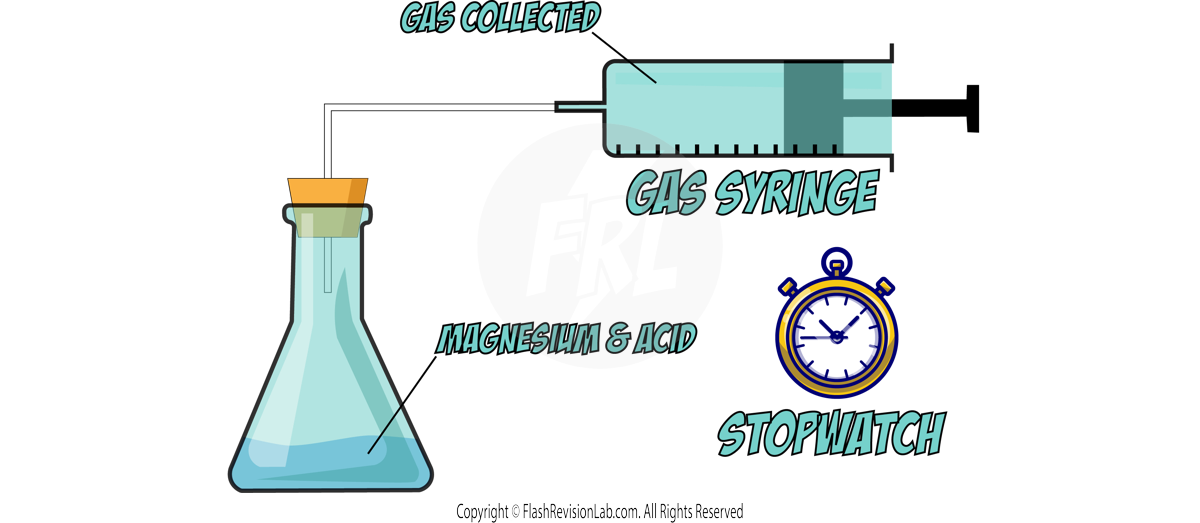
- Pour a known volume of HYDROCHLORIC ACID (HCl) into a CONICAL FLASK.
- Add a piece of MAGNESIUM RIBBON with a known mass to the flask.
- Quickly connect the flask to the GAS SYRINGE setup to capture the gas produced.
- Start the STOPWATCH and record the VOLUME OF GAS produced every 10 SECONDS.
- Once the reaction is complete, repeat the steps with DIFFERENT CONCENTRATIONS.
Results:
Plot a graph with TIME on the x-axis and VOLUME OF GAS on the y-axis to visualise the results.
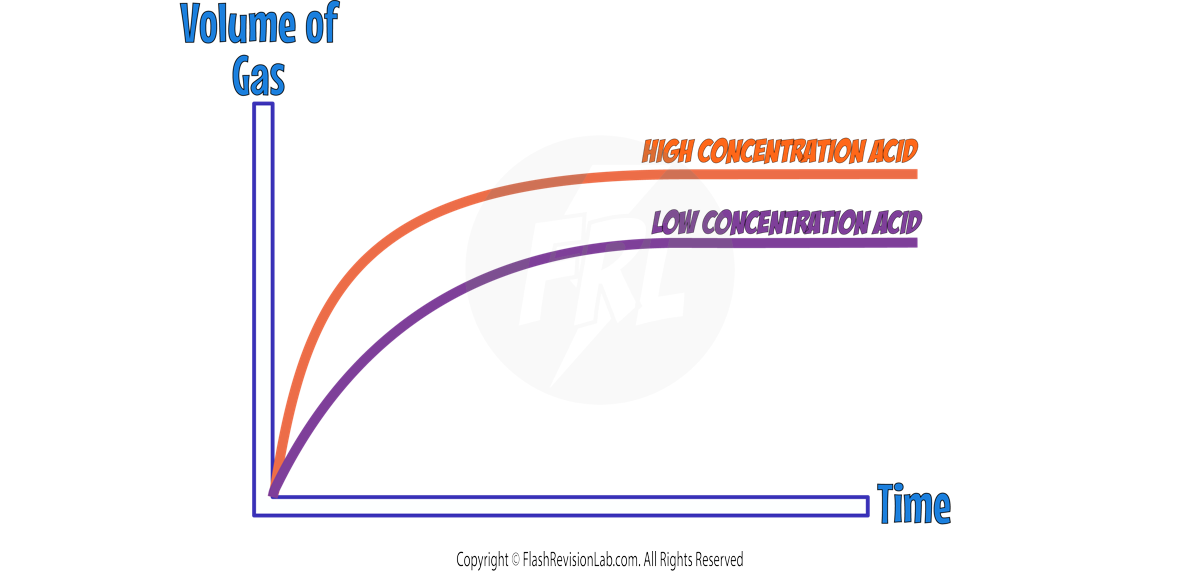
- The reaction with a HIGHER CONCENTRATION will result in a graph with a STEEPER GRADIENT which levels off EARLIER.
- This shows that it has a FASTER RATE of reaction and the reaction is COMPLETED EARLIER.
- The graph with the HIGHER CONCENTRATION also levels off HIGHER which shows that MORE GAS has been given off. This is because a more concentrated acid has MORE REACTANT PARTICLES in the same volume which results in MORE PRODUCT being formed.

Factors Affecting Reaction Rates
There are FOUR factors that affect the rate of a reaction:
1. Temperature
2. Pressure/Concentration
3. Surface Area
4. Using a Catalyst
Collision Theory
To understand the factors affecting the RATE of a reaction, you can think of all the reactant and products as PARTICLES.
Let's consider the following reaction:

At the start of the reaction, only REACTANT particles are present. These particles are constantly moving around the container and COLLIDE with each other.
Most of these collisions are UNSUCCESSFUL and do NOT result in a REACTION.
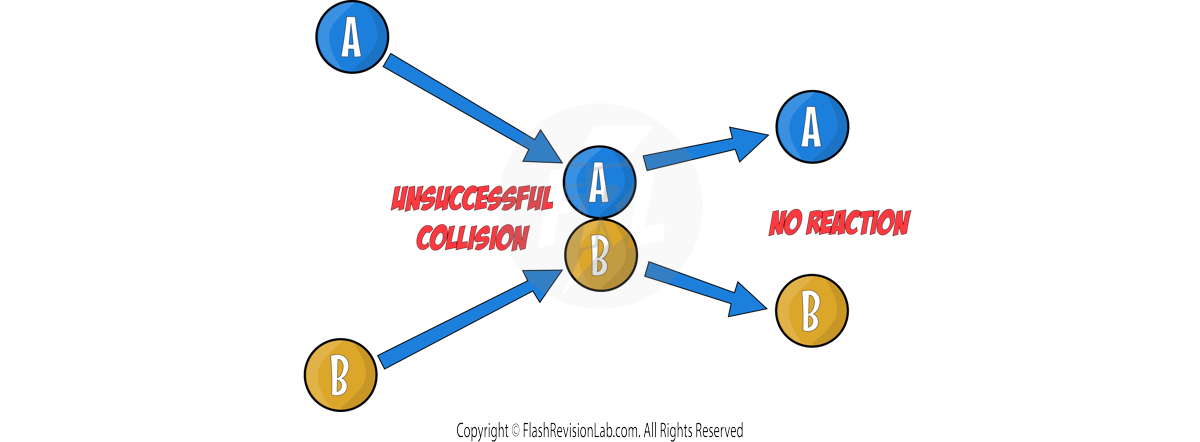
If two particles COLLIDE with an energy GREATER than the ACTIVATION ENERGY, they REACT and form the product particles. This is known as a SUCCESSFUL COLLISION.
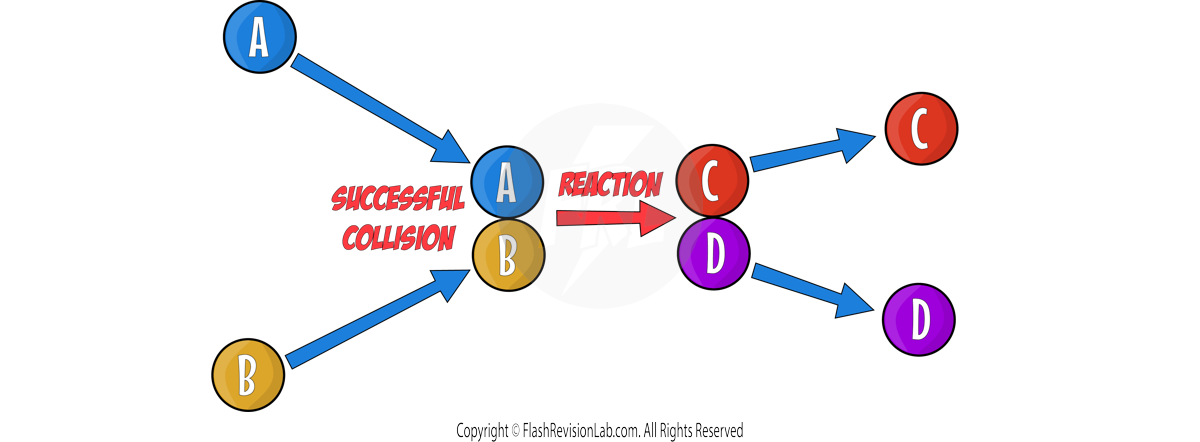
ACTIVATION ENERGY: The minimum amount of energy particles need to collide with in order to react.
The rate of reaction INCREASES when:
- There are MORE FREQUENT COLLISIONS.
- There are MORE ENERGETIC COLLISIONS.
Temperature
- As TEMPERATURE increases, the KINETIC ENERGY of particles INCREASES. This means particles move with GREATER SPEEDS, leading to MORE FREQUENT COLLISIONS.
- Furthermore, the collisions are MORE ENERGETIC, meaning a greater proportion of the collisions have an energy greater than the ACTIVATION ENERGY.
- This means the rate of reaction is FASTER.
Concentration and Pressure
- The CONCENTRATION of reactants in a solution or the PRESSURE of reacting gases affect the number of particles in a given volume.
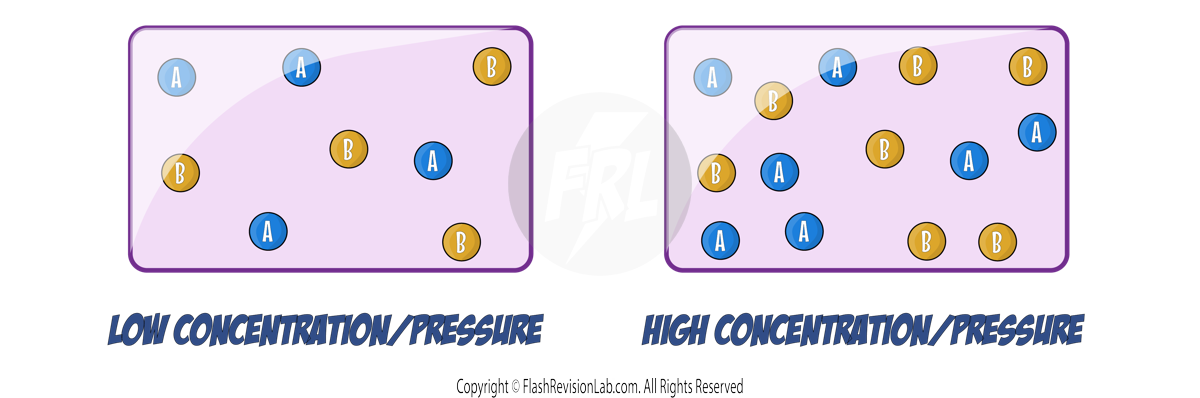
- A higher CONCENTRATION or PRESSURE results in particles being CLOSER together, meaning there are MORE FREQUENT COLLISIONS, which INCREASES the rate of reaction.
Surface Area
- You can increase the surface area to volume ratio of a reactant by breaking it down into SMALLER PARTICLES (such as powder).
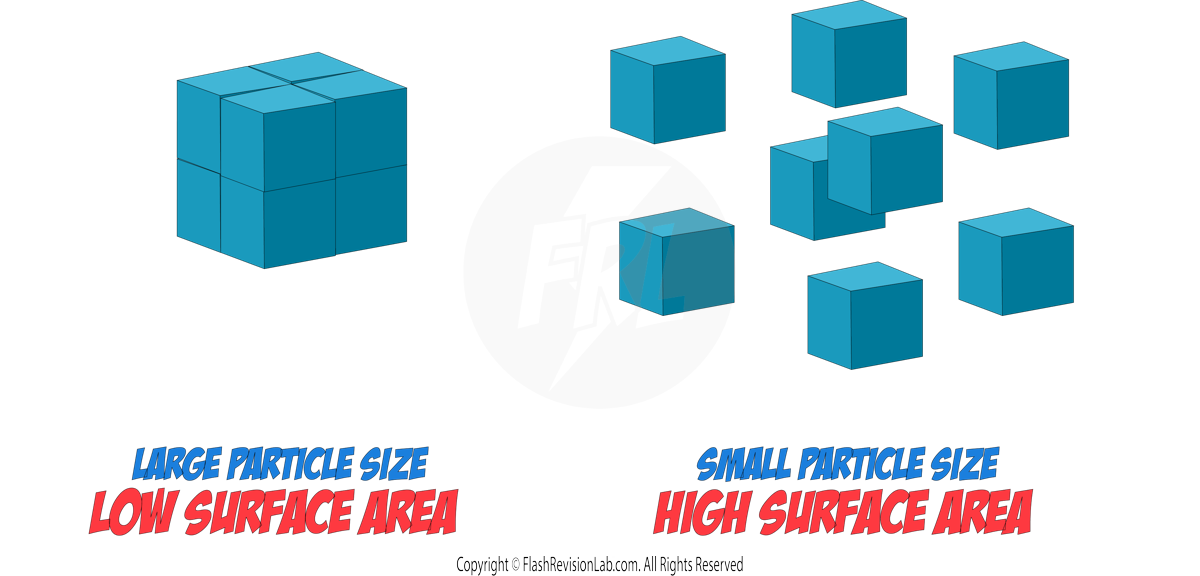
- By increasing the SURFACE AREA, we expose more reactant particles to possible collisions.
- Smaller particle sizes or finely divided materials present a larger SURFACE AREA to volume ratio, allowing for MORE FREQUENT COLLISIONS and a faster reaction rate as compared to larger chunks of the same substance.
Catalysts
- CATALYSTS are substances that INCREASE the reaction RATE without being USED UP in the reaction.
- This means they will NOT appear in the chemical equations.
- Catalysts are SPECIFIC, meaning different reactions need different catalysts.
- ENZYMES are examples of CATALYSTS that increase the rate of biological reactions.
- Catalysts work by providing an ALTERNATIVE REACTION PATHWAY with a LOWER ACTIVATION ENERGY.
- You can see activation energies on REACTION PROFILES:
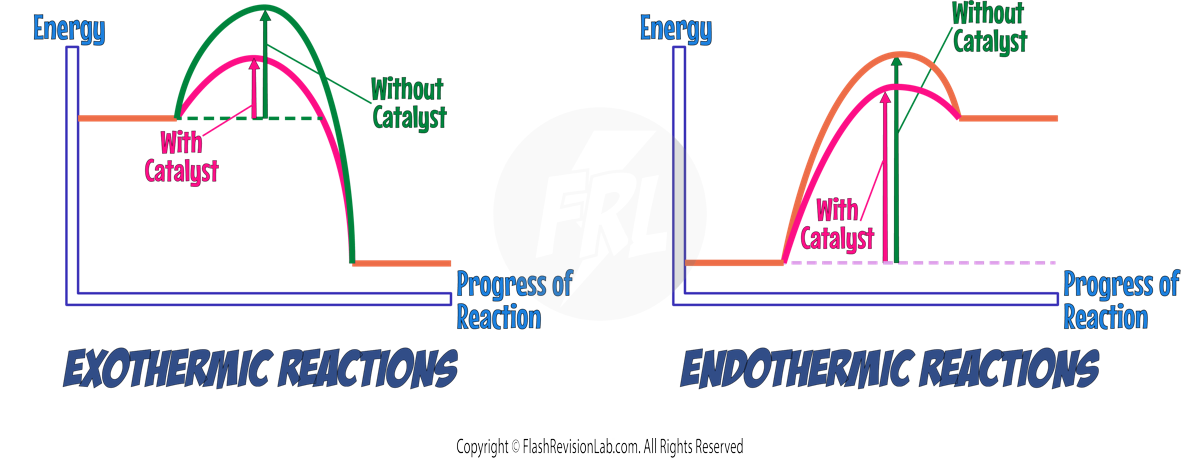
The ACTIVATION ENERGY is represented by the height from the REACTANTS to the PEAK of the graph.
You can see that the activation energy for the reactions WITH a catalyst is LOWER.
This means a greater proportion of the particles will have an energy GREATER than the ACTIVATION ENERGY, meaning there will be MORE FREQUENT COLLISIONS, which will INCREASE the rate of the reaction.
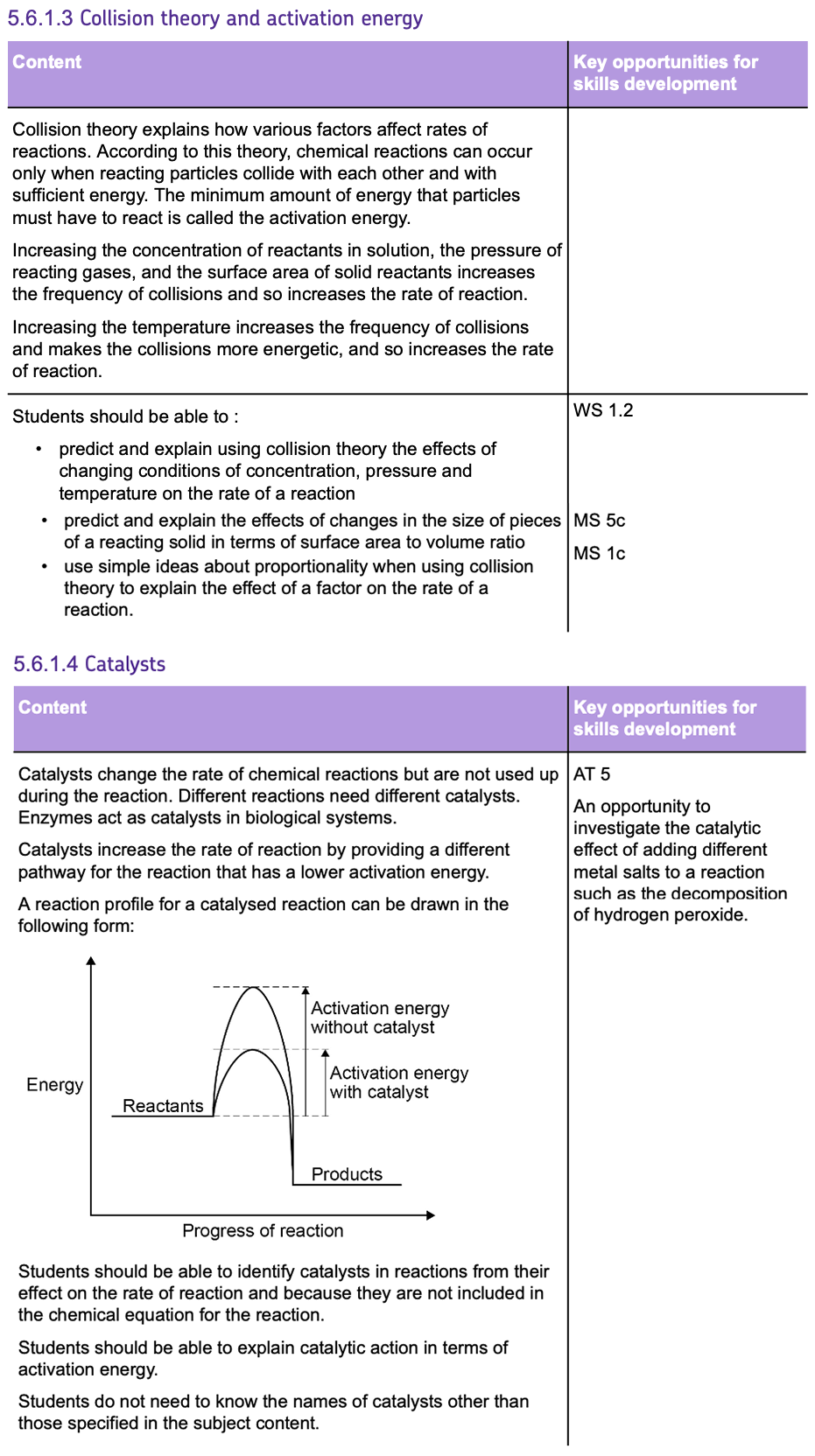
Reversible Reactions
- REVERSIBLE REACTIONS occur when products can react to form the original reactants again.
- You can represent a reversible reaction with a DOUBLE ARROW.

- The FORWARD REACTION represents the REACTANTS turning into PRODUCTS (from left to right).
- The REVERSE REACTION represents the PRODUCTS turning into REACTANTS (from right to left).
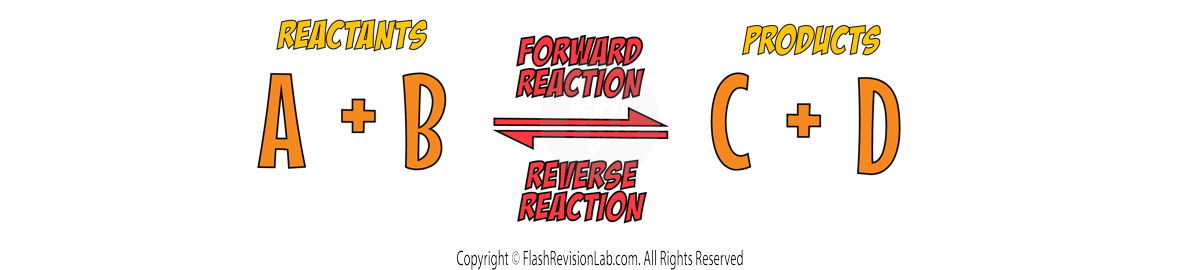
You can change the direction of a REVERSIBLE REACTION by changing the CONDITIONS.
For example, Ammonium Chloride:

If you HEAT the AMMONIUM CHLORIDE, it will decompose to form AMMONIA and HYDROGEN CHLORIDE in the FORWARD REACTION.

If you COOL the products AMMONIA and HYDROGEN CHLORIDE, the REVERSE REACTION would take place and the AMMONIUM CHLORIDE would be formed.

ENERGY CHANGES IN REVERSIBLE REACTIONS
All reactions involve an ENERGY CHANGE. They can either GIVE OUT energy (EXOTHERMIC) or TAKE IN energy (ENDOTHERMIC).
In REVERSIBLE reactions, forward and reverse reactions have OPPOSITE ENERGY CHANGES:

If the forward reaction is EXOTHERMIC, then the reverse reaction must be ENDOTHERMIC and vice versa. The SAME AMOUNT of energy is transferred in each case.
Example:
This can be demonstrated with HYDRATED and ANHYDROUS SALTS.
HYDRATED SALTS are salts that CONTAIN WATER. They have water molecules bonded to their structures. They are represented with a DOT in between the salt and the surrounding water.
For example, HYDRATED COPPER SULFATE can be written as:

It has a BLUE colour.
ANHYDROUS SALTS are salts that have LOST all their water. This would just be written as the salt on its own:

This has a WHITE colour.
The reaction involving HYDRATED and ANHYDROUS copper sulfate is REVERSIBLE and can be written as:
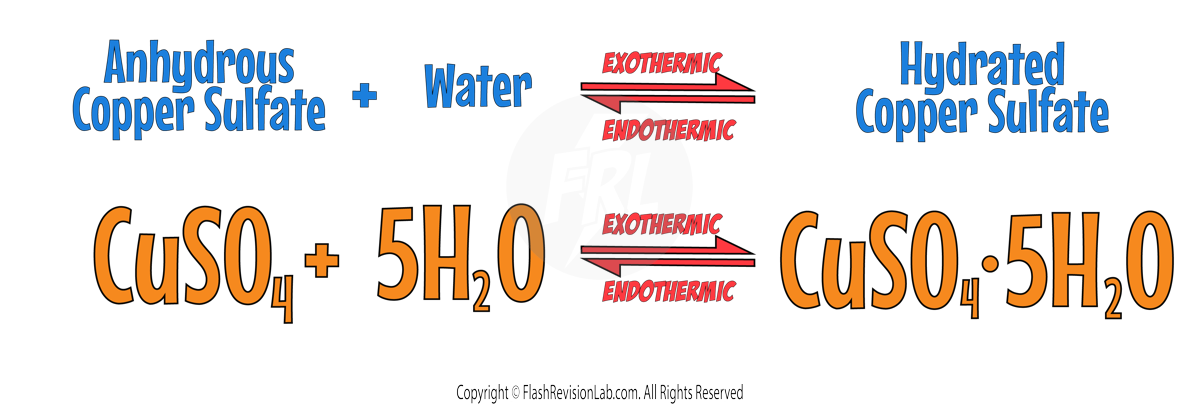
In this reaction, the forward reaction is EXOTHERMIC and the reverse reaction is ENDOTHERMIC.
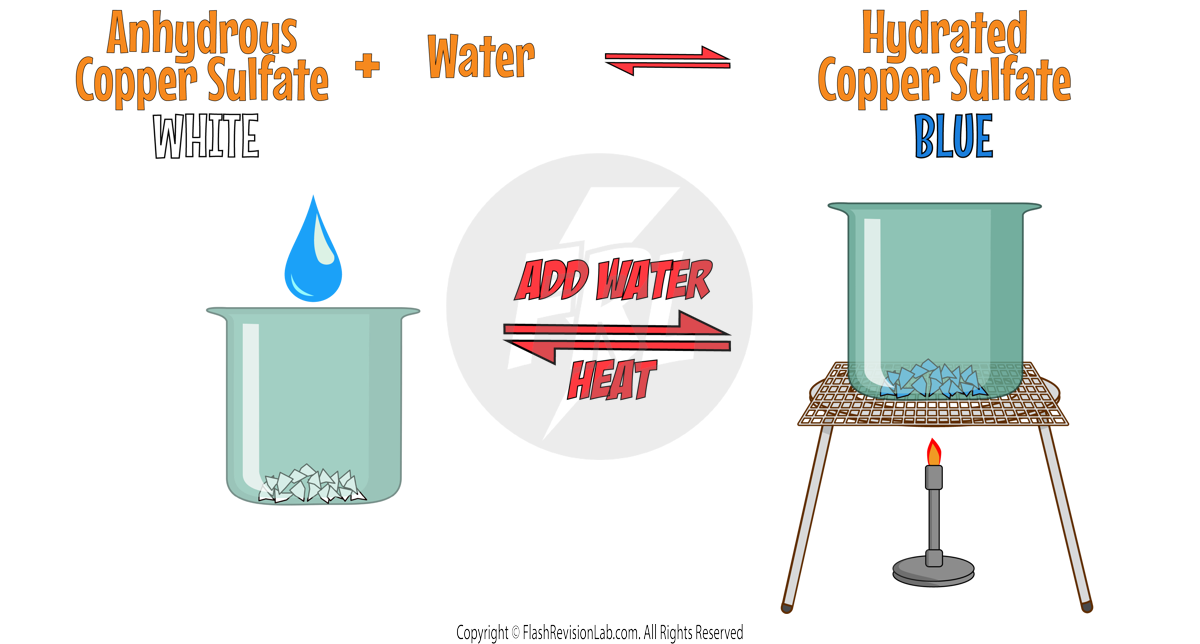
You can turn ANHYDROUS Copper Sulfate into HYDRATED Copper Sulfate by ADDING WATER. This causes it to change colour from WHITE to BLUE.
You can turn HYDRATED Copper Sulfate into ANHYDROUS Copper Sulfate by HEATING IT. This causes it to change colour from BLUE to WHITE.
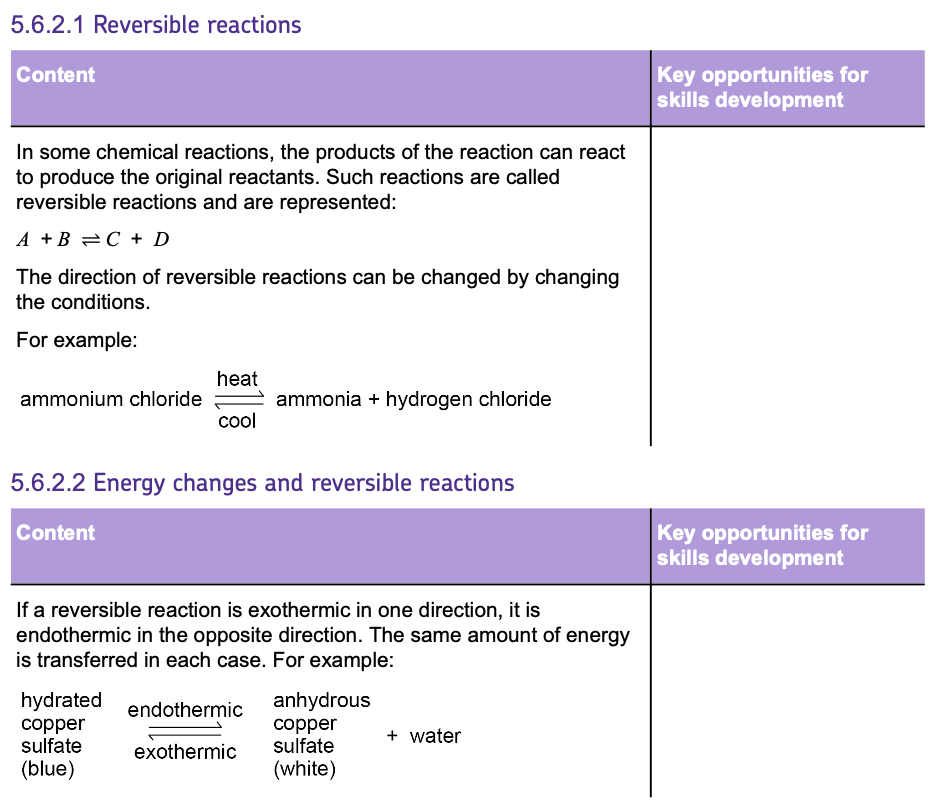
Equilibrium
Equilibrium occurs when you have a REVERSIBLE REACTION in a CLOSED SYSTEM.
A CLOSED SYSTEM is a system where nothing can ENTER or LEAVE. E.g. a conical flask with a rubber bung closing it.
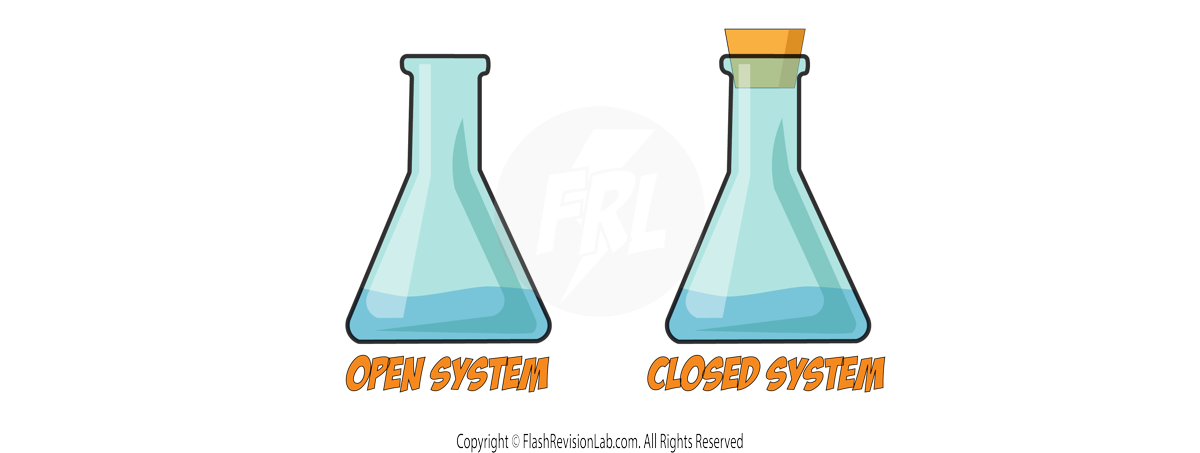
At the start of any reaction, only REACTANT particles are present. As these collide and REACT, they form PRODUCT particles.
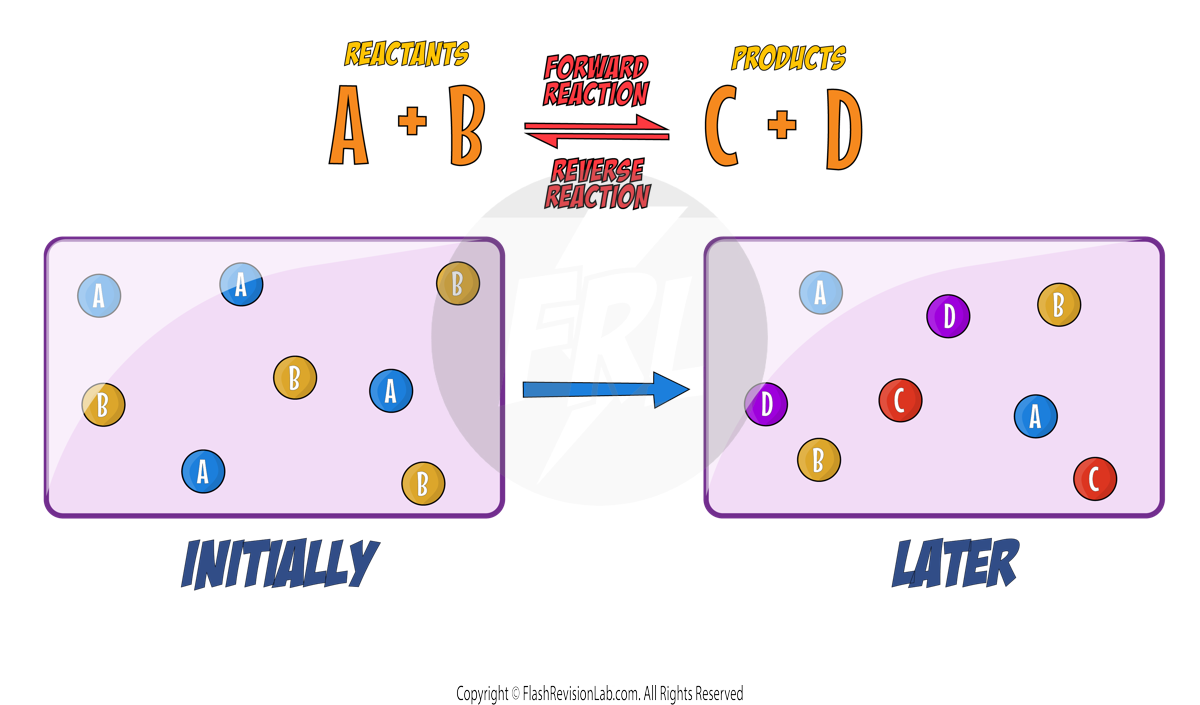
In normal reactions, the amount of reactants would DECREASE to ZERO at the end of the reaction, and the amount of products would INCREASE to a MAXIMUM.
However this does NOT happen in a reversible reaction.
The PRODUCTS can also react to reform the REACTANTS in the REVERSE reaction.
This means there are TWO reactions occurring in the closed system:

When the FORWARD and REVERSE reactions both occur at the SAME RATE, we say the reaction is in EQUILIBRIUM.
At this point, the AMOUNTS of reactants and products STAY THE SAME.
Shifting the Equilibrium
You can change the RELATIVE AMOUNTS of reactants and products in a reaction at equilibrium by changing the CONDITIONS of the reaction. This is known as SHIFTING the POSITION of equilibrium and you can shift it in TWO WAYS:
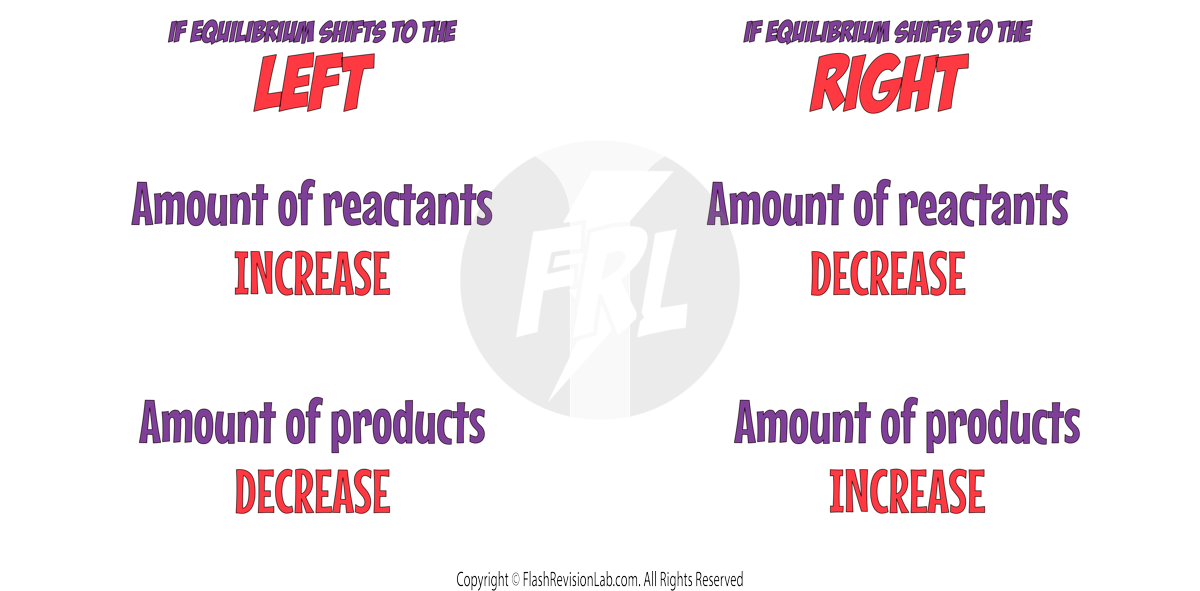
Shifting the equilibrium to the LEFT will cause the relative amounts of the REACTANTS to INCREASE and the PRODUCTS to DECREASE.
Shifting the equilibrium to the RIGHT will cause the relative amounts of the REACTANTS to DECREASE and the PRODUCTS to INCREASE.
FACTORS THAT SHIFT EQUILIBRIUM
There are THREE factors that can shift the equilibrium and affect its POSITION:
1. TEMPERATURE
2. PRESSURE
3. CONCENTRATION
To work out the effect that CHANGING one of these FACTORS has on a system at equilibrium, you can use LE CHATELIER'S PRINCIPLE.
Le Chatelier’s Principle
Le Chatelier’s principle states that if a CHANGE is made to the conditions of a system at EQUILIBRIUM, the system will respond and SHIFT to COUNTERACT the change.
For example, if you have a system at equilibrium and you heat it to INCREASE the temperature, the equilibrium will respond in a way to DECREASE the temperature back to normal.
Here are some more examples:
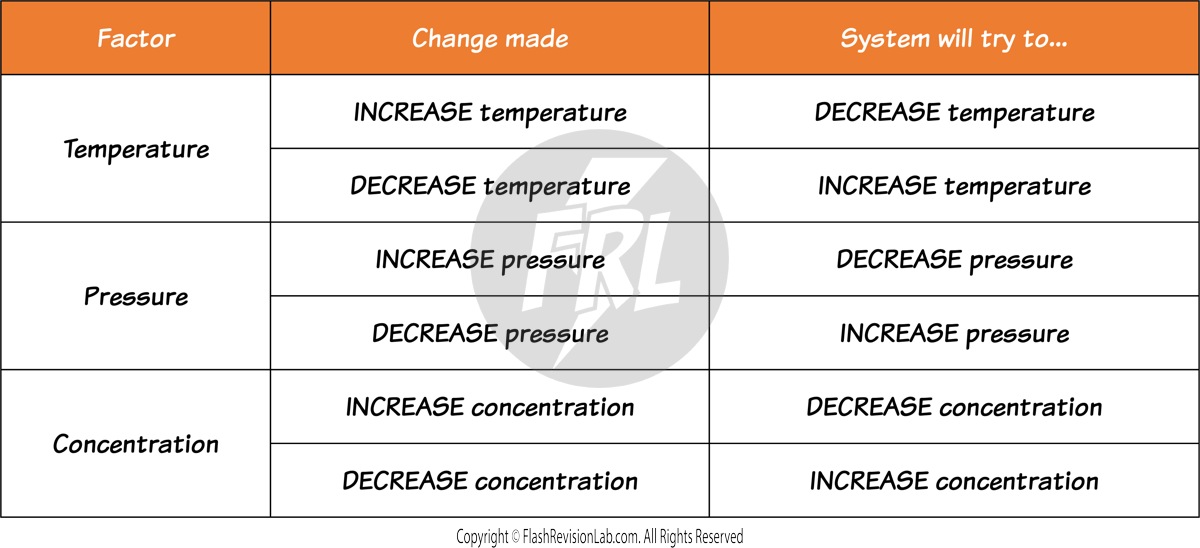
TEMPERATURE
If you change TEMPERATURE, the direction that the equilibrium shifts depends on whether the forward reaction is EXOTHERMIC or ENDOTHERMIC.
ENDOTHERMIC reactions show a DECREASE in temperature, while EXOTHERMIC reactions show an INCREASE in temperature.
This means that if you INCREASE the temperature of a system at equilibrium, it will try to COUNTERACT the change by DECREASING the temperature, so it will shift to the ENDOTHERMIC side of the reaction. The opposite will happen if you DECREASE the temperature.
This shifting of equilibrium can affect the amount of reactants and products in the system.
For an example where the FORWARD reaction is EXOTHERMIC:
If the FORWARD reaction is ENDOTHERMIC, the opposite would happen:
PRESSURE
For gaseous reactions, if you change PRESSURE, the direction that the equilibrium shifts depends on the NUMBER OF MOLECULES (moles) on either side of the equation. These are the THREE scenarios you can have:
The more MOLECULES you have in a closed system, the GREATER the PRESSURE.
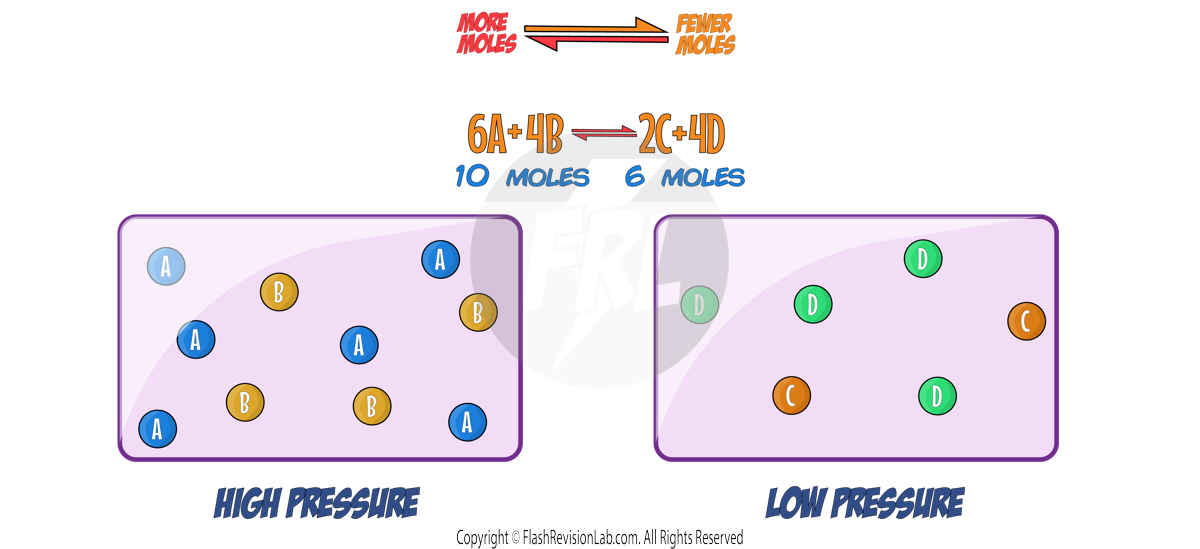
This means if you INCREASE the pressure of a system at equilibrium, it will try to COUNTERACT the change by DECREASING the pressure, so it will shift to the side with FEWER MOLECULES. The opposite will happen if you DECREASE the pressure.
This shifting of equilibrium can affect the amount of reactants and products in the system.
For an example where there are MORE MOLES on the LEFT:
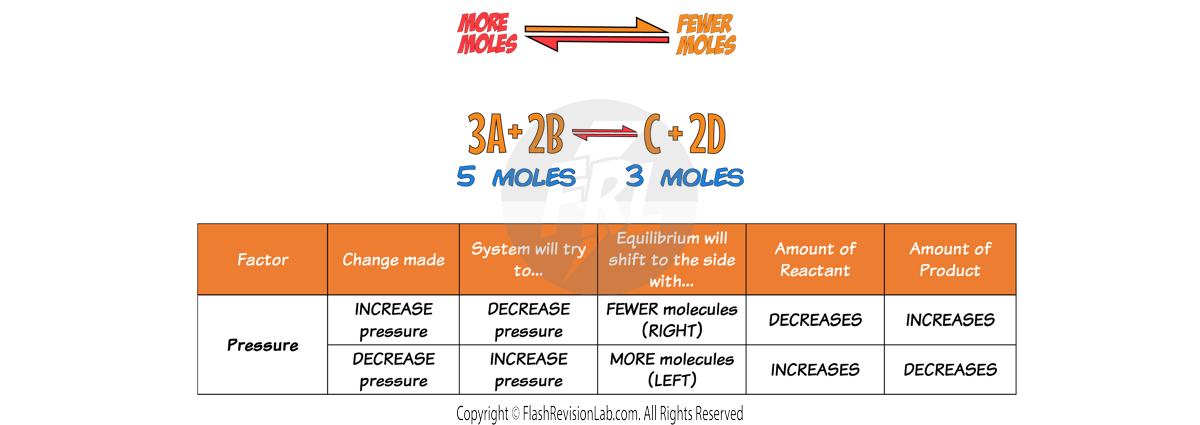
If there are MORE MOLES on the RIGHT, the opposite would happen:
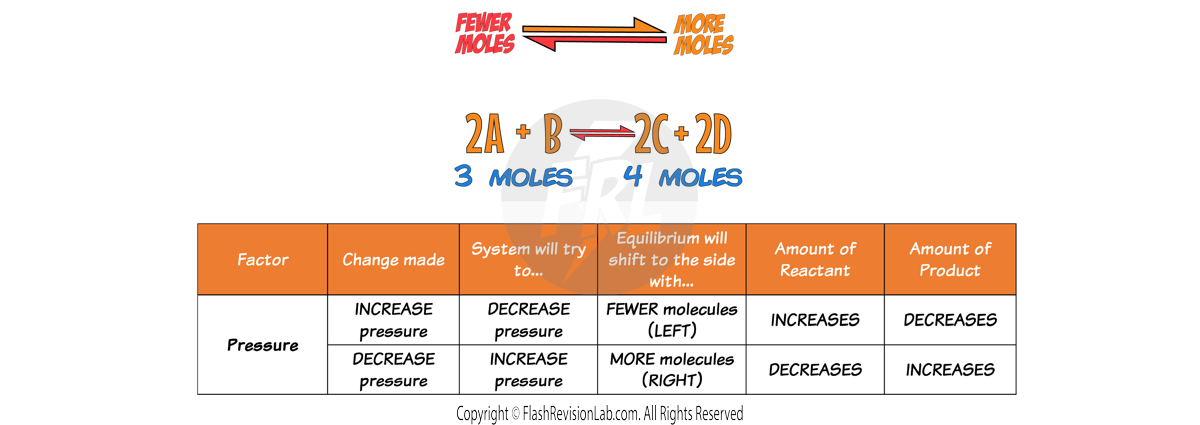
If there are the SAME number of moles on the left and right, NOTHING HAPPENS.
CONCENTRATION
If you were to change the CONCENTRATION, you will have disrupted the equilibrium balance, meaning the reaction is no longer in equilibrium for a short amount of time.
When this happens, the reaction RESPONDS and gets itself back into equilibrium and COUNTERACTS the change that was made to it.
You can predict the direction the equilibrium shifts for the short amount of time by knowing whether the concentration that changes is a REACTANT or PRODUCT.
Here are all the possible scenarios:
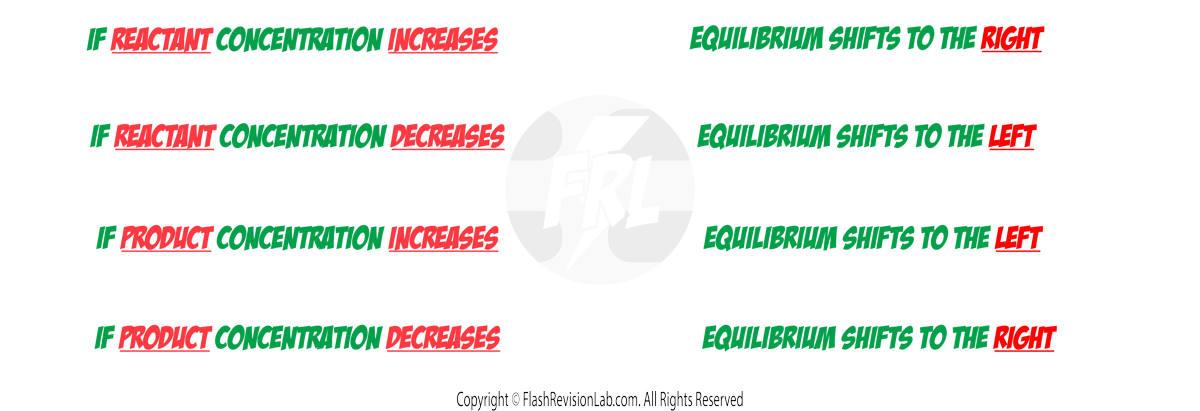
Crude Oil and Fractional Distillation
THE FORMATION OF CRUDE OIL
- CRUDE OIL is a type of FOSSIL FUEL formed from ancient biological materials like PLANKTON which were buried in mud and subjected to high pressures and temperatures for millions of years.
- It can now be found in rocks and which can be drilled to extract it.
- It is considered a FINITE RESOURCE (NON-RENEWABLE FUEL) because it takes over MILLIONS OF YEARS to form.
- Crude oil is a MIXTURE of many different compounds, most of which are HYDROCARBONS.
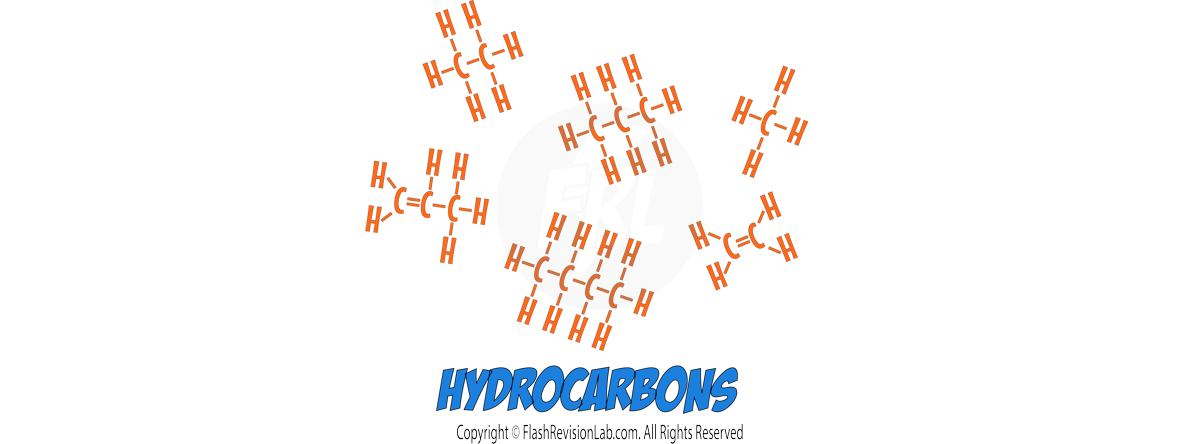
- Hydrocarbons are COMPOUNDS made of HYDROGEN and CARBON atoms ONLY.
- The components in the crude oil can be separated using FRACTIONAL DISTILLATION.
FRACTIONAL DISTILLATION OF CRUDE OIL
FRACTIONAL DISTILLATION is the method used to separate the MIXTURE OF HYDROCARBONS in crude oil.
The process includes:
HEATING AND VAPORISATION
- Crude oil is HEATED until most hydrocarbons turn into GAS.
- These gases enter a FRACTIONATING COLUMN where there's a TEMPERATURE GRADIENT (hot at the bottom, cooler as you go up).
SEPARATION BY BOILING POINTS
- LONGER HYDROCARBONS with HIGHER boiling points CONDENSE back into liquids at the LOWER part of the column.
- SHORTER HYDROCARBONS with LOWER boiling points travel further up and condense at higher levels.
- The different hydrocarbon fractions are collected at various levels, each with a similar boiling points and HYDROCARBON LENGTH.
PRODUCTS FROM DISTILLATION
- The FRACTIONS produced from the fractional distillation include LPG, Petrol, Kerosene, Diesel, Heavy Fuel Oil and Bitumen.
- The fractions can then be processed to produce fuels and feedstock for the PETROCHEMICAL INDUSTRY.
- Many useful materials on which modern life depends on are produced by the petrochemical industry, such as SOLVENTS, LUBRICANTS, POLYMERS and DETERGENTS.
BOILING POINTS OF HYDROCARBONS
Fractional distillation relies on the differences in boiling points to separate components. The TEMPERATURE GRADIENT is crucial as it allows the hydrocarbons to condense at the right levels, to ensure effective separation.
The SHORTER CHAINED hydrocarbons like the ones in LPG and Petrol have a LOWER BOILING POINT because there are WEAKER INTERMOLECULAR FORCES between the molecules which don’t need a lot of energy to break.
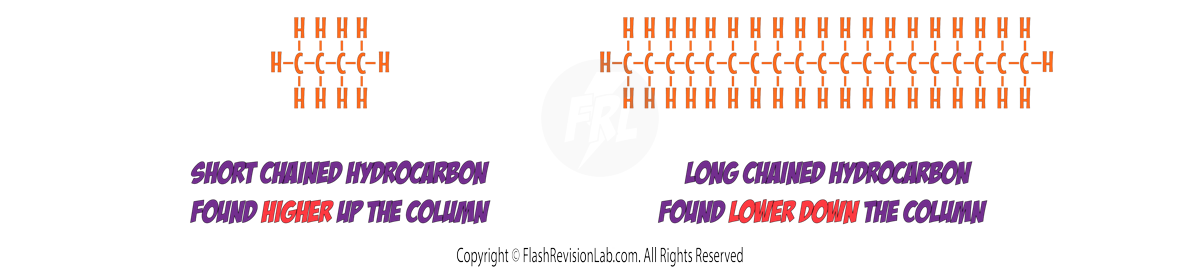
This means their BOILING POINTS are LOWER, so they are collected at HIGHER LEVELS in the fractionating column because the temperatures further up are COOLER.
The opposite can be said for the LONGER CHAINED HYDROCARBONS.
Hydrocarbons
Hydrocarbons are compounds made up of CARBON and HYDROGEN atoms ONLY. They have different properties that can change with the size of their molecules. These properties include:
1. BOILING POINT:
This is the TEMPERATURE at which a substance changes from a liquid to a gas.
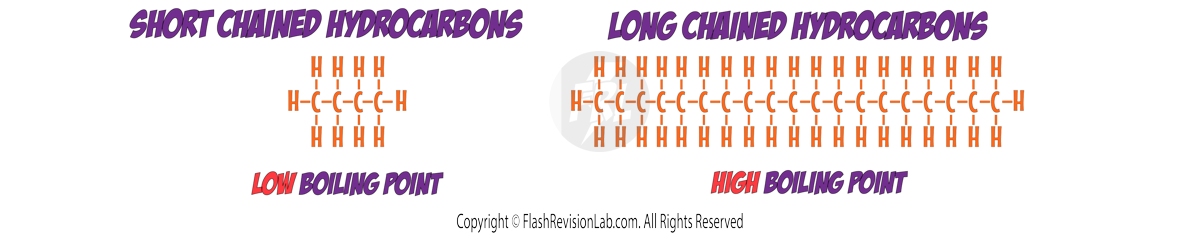
As the molecular size increases, the INTERMOLECULAR FORCES between the molecules INCREASES. This means that MORE ENERGY is needed to break these forces, resulting in HIGHER BOILING POINTS.
2. VISCOSITY:,
Viscosity refers to how THICK a liquid is in comparison to water.
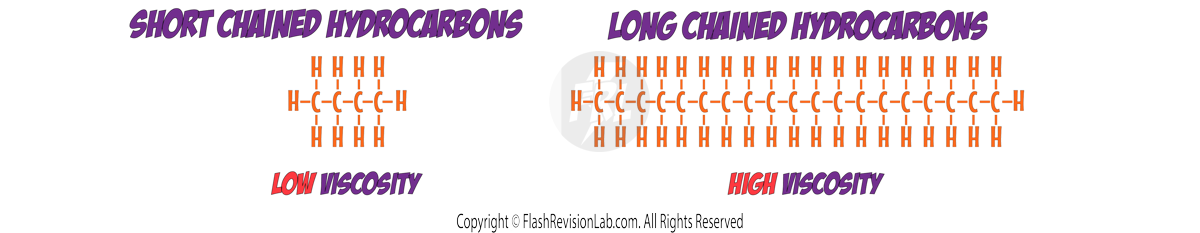
With hydrocarbons, the LARGER the molecules, the HIGHER the viscosity.
3. FLAMMABILITY:
This is the ability of a substance to CATCH FIRE or IGNITE.
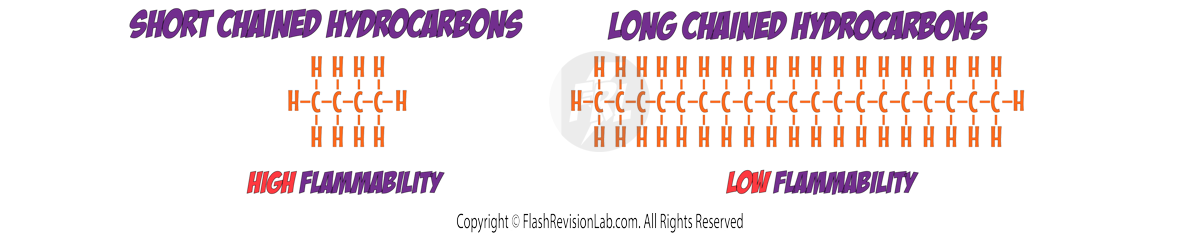
SMALLER hydrocarbon molecules tend to be MORE FLAMMABLE compared to larger ones.
HYDROCARBONS AS FUELS
When hydrocarbons are used as FUELS, these properties affect how they are utilised. For example:
- Boiling Point: Hydrocarbons with LOW BOILING POINTS tend to be GASES at room temperature so can easily be COMPRESSED and stored in canisters.
- Viscosity: A high viscosity might affect how easily a fuel FLOWS through pipes.
- Flammability: A fuel that ignites easily may need more SAFTEY PRECAUTIONS when being used.
During COMBUSTION, which is the process of burning, hydrocarbons RELEASE ENERGY.
Complete combustion means that the hydrocarbon reacts with OXYGEN to form CARBON DIOXIDE and WATER. Here is an example:
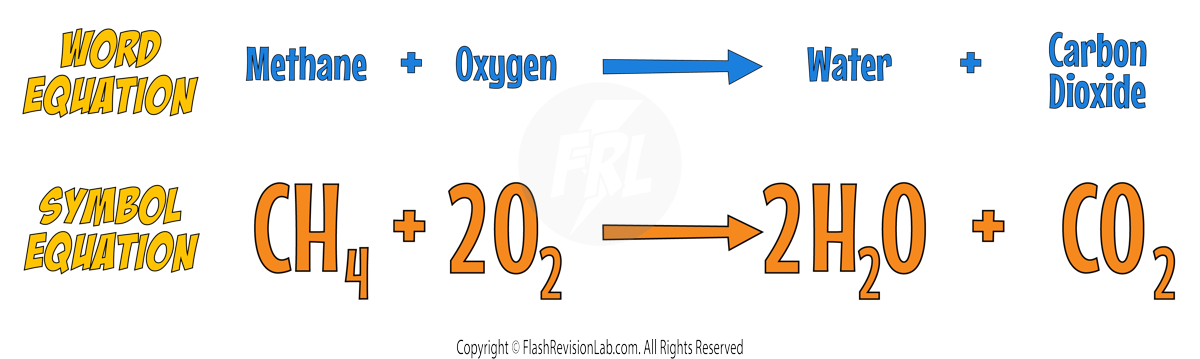
Alkanes
Alkanes are known as SATURATED HYDROCARBONS.
- Hydrocarbons: They consist of HYDROGEN and CARBON atoms ONLY.
- Saturated: They ONLY contain single bonds.
They are an example of a HOMOLOGOUS SERIES - A group of compounds that have similar structures with the SAME GENERAL FORMULA.
The general formula for alkanes is CnH2n+2.
The general formula helps you figure out the formula of ANY ALKANE, just by knowing the number of carbon atoms in it.
The first four alkanes that you need to know are:
- METHANE (CH₄)
- ETHANE (C₂H₆)
- PROPANE (C₃H₈)
- BUTANE (C₄H₁₀)
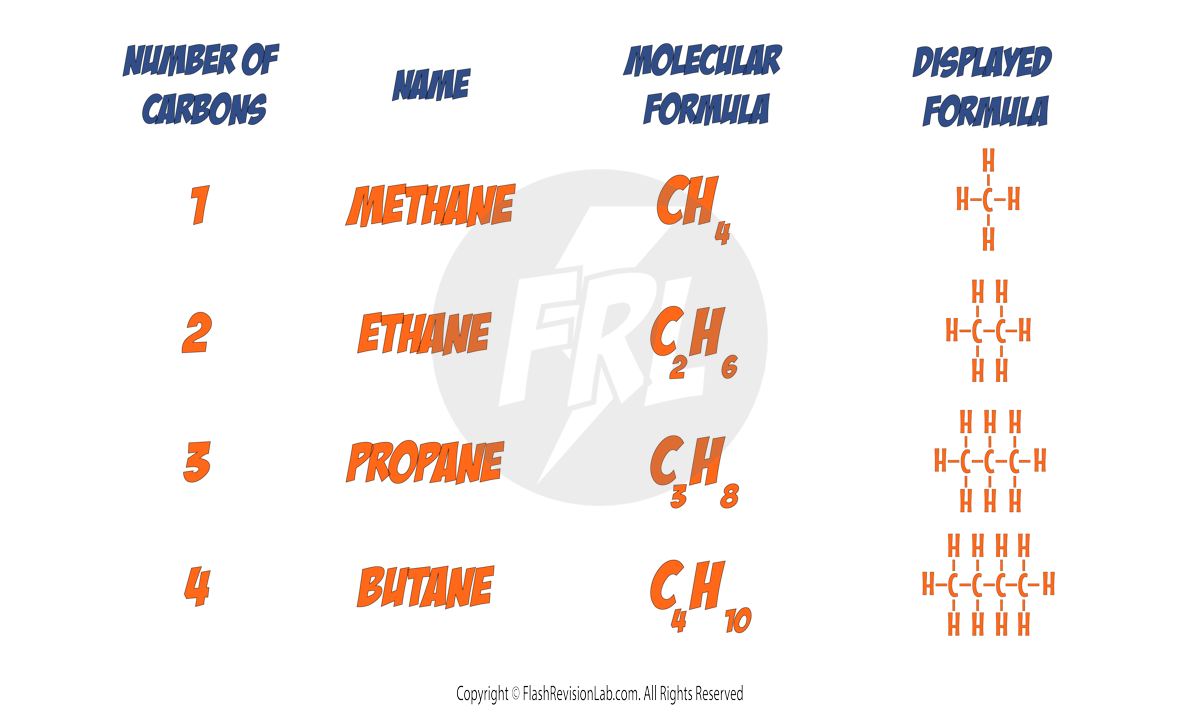
In these structures, each carbon atom forms FOUR bonds, and each hydrogen atom forms ONE bond.
The GENERAL FORMULA (CnH2n+2) can be used to determine the MOLECULAR FORMULA.
For instance, if you had 5 carbons (n = 5) for an alkane, its molecular formula would be C₅H₁₂.

Cracking and Alkenes
CRACKING
CRACKING is a process where LONG HYDROCARBONS are broken down into SMALLER, more USEFUL molecules.
There's a HIGH DEMAND for fuels with small molecules, making the products of cracking very important as FUELS.
There are TWO types of cracking:
1. CATALYTIC CRACKING:
This involves heating the hydrocarbon to vaporise it.
The VAPOUR is then passed over a HOT POWDERED ALUMINIUM OXIDE CATALYST.
This causes the long chained molecules to break down on the surface of the catalyst.
2. STEAM CRACKING:
This involves vaporising the long hydrocarbons and MIXING them with STEAM.
The mixture is then HEATED to very high temperatures to break down the molecules.
Here is an example of a cracking reaction:
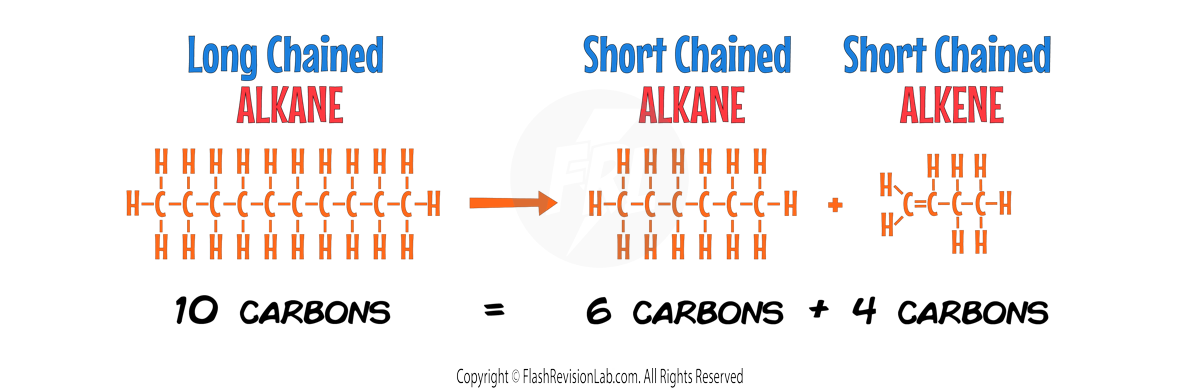
The PRODUCTS OF CRACKING include:
- ALKANES: Saturated hydrocarbons with single bonds between carbon atoms.
- ALKENES: Unsaturated hydrocarbons with at least one double bond between carbon atoms.
ALKENES
ALKENES are UNSATURATED hydrocarbons, which means they have at least one CARBON TO CARBON DOUBLE BOND.
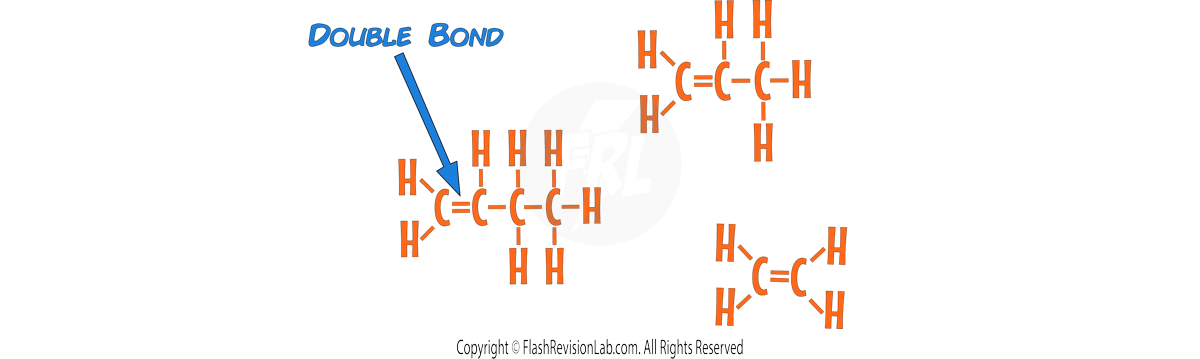
ALKENES are more REACTIVE than alkanes. This means they can easily react with halogens such as BROMINE.
Test for Alkenes:
You can identify an alkene by adding BROMINE WATER to it.
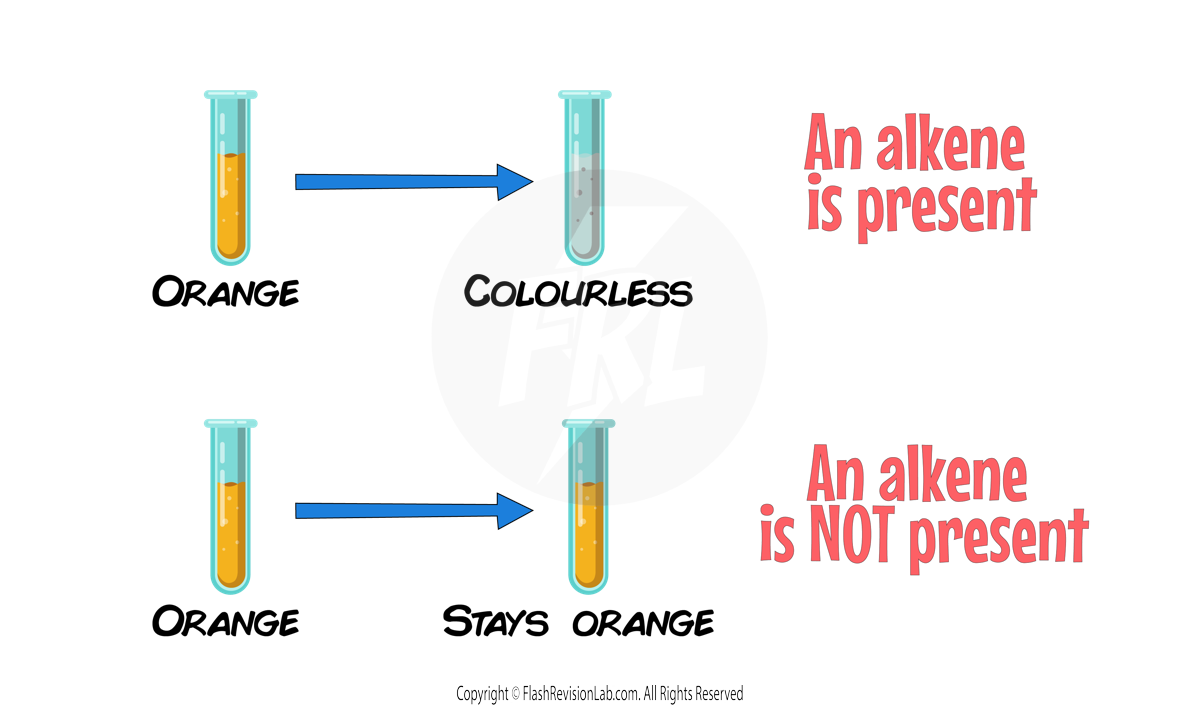
If the ORANGE colour of the bromine water TURNS COLOURLESS, then the solution is an ALKENE.
USES OF ALKENES:
- To produce POLYMERS.
- As STARTING MATERIALS for making other chemicals.
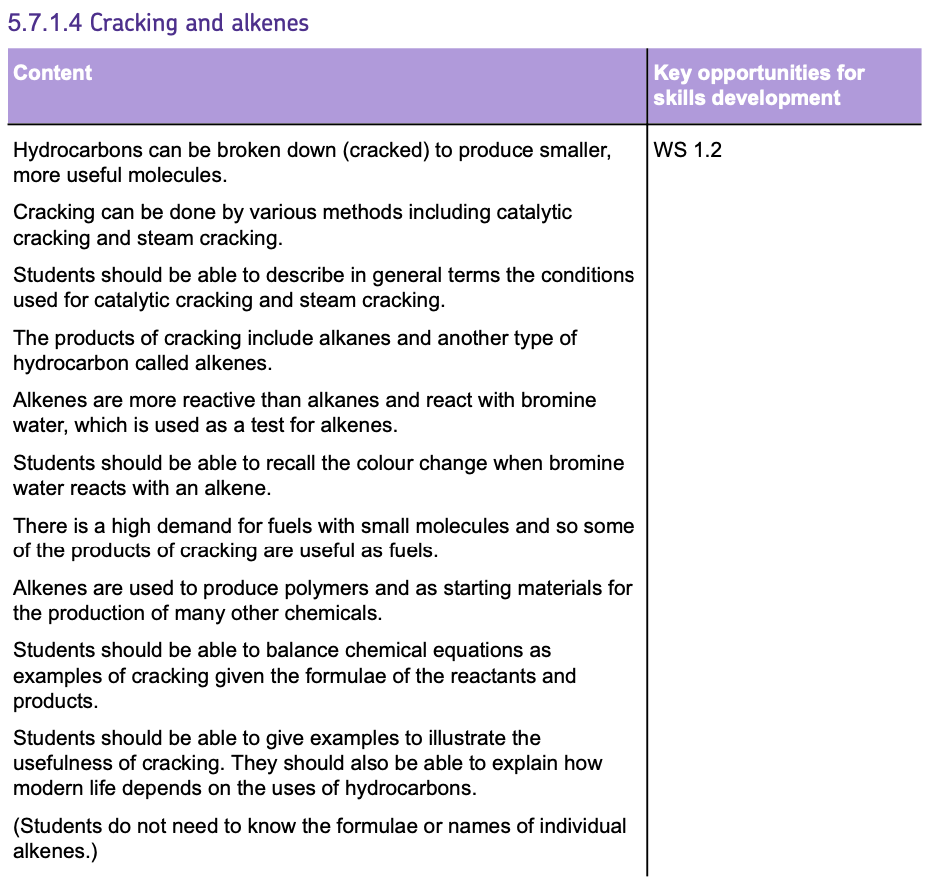
Pure Substances and Formulations
PURE SUBSTANCES
In everyday life, a pure substance can mean a substance that has had NOTHING ADDED to it, so it is UNADULTERATED and in its natural state, e.g. pure milk.
In Chemistry, a pure substance is a single element or compound, NOT mixed with any other substance.
You can tell whether a substance is pure or not by checking its MELTING or BOILING POINT.
Pure substances will always have a specific melting or boiling point, so if a substance deviates from the known boiling points, there are IMPURITIES present.
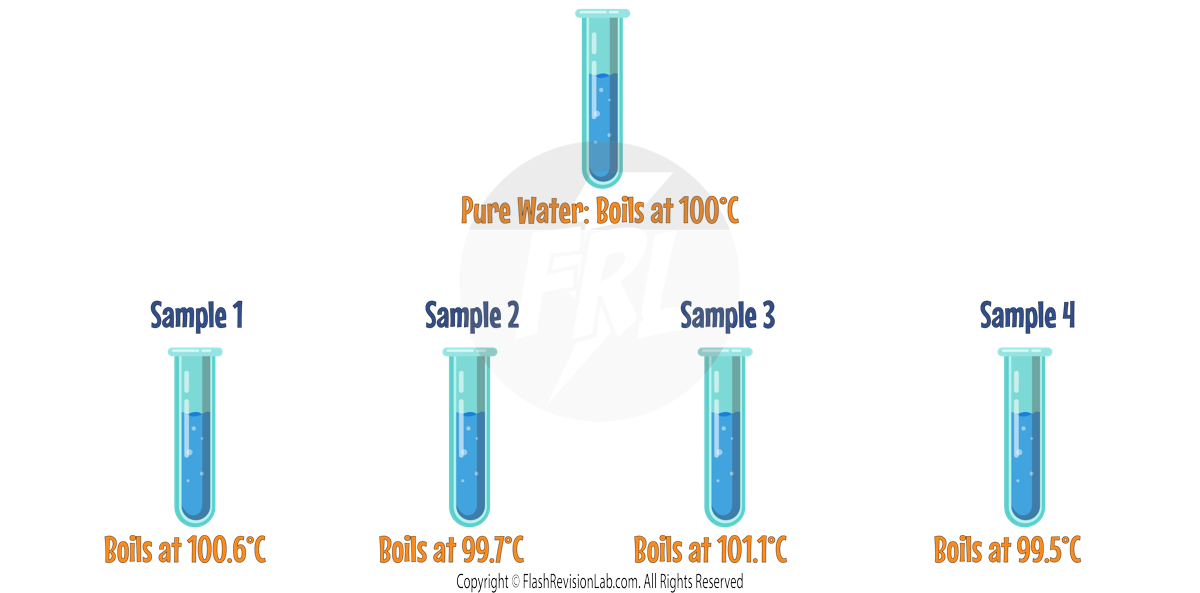
For example, SAMPLE 2 would be the PUREST out of the 4 samples, as it has the closest boiling point to PURE water.
FORMULATIONS
A formulation is a mixture that has been carefully designed as a USEFUL PRODUCT. These are complex mixtures in which EACH CHEMICAL has a particular PURPOSE.
For example in PAINT, the components mixed together include:

PIGMENT: Provides the COLOUR.
BINDER: Creates a film to HOLD the pigment in place.
SOLVENT: DISSOLVES the components and alters the VISCOSITY.
Another example would be in TABLETS, which would have many important components such as the active ingredient, binders and coatings.
Formulations are made by mixing the components in carefully MEASURED quantities to ensure that the product has the required properties.
Other examples of formulations found in everyday products include:
FUELS, CLEANING AGENTS, PAINTS, MEDICINES, ALLOYS, FERTILISERS, and FOODS.
Chromatography
CHROMATOGRAPHY is a technique used to SEPARATE and IDENTIFY components in a mixture.
It involves TWO phases:

1. MOBILE PHASE:
The phase where the MOLECULES MOVE. This is usually a LIQUID or a GAS.
E.g. In PAPER chromatography, this would be the WATER or SOLVENT used.
2. STATIONARY PHASE:
The phase where the molecules DON'T move, often a SOLID or THICK LIQUID.
E.g. In PAPER chromatography, this would be the PAPER.
How Chromatography Works
- During the process, the solvent moves up and carries the components in the mixture with it.
- The distance a component moves up depends on the DISTRIBUTION between the MOBILE and STATIONARY phases.
- This is affected by TWO things:
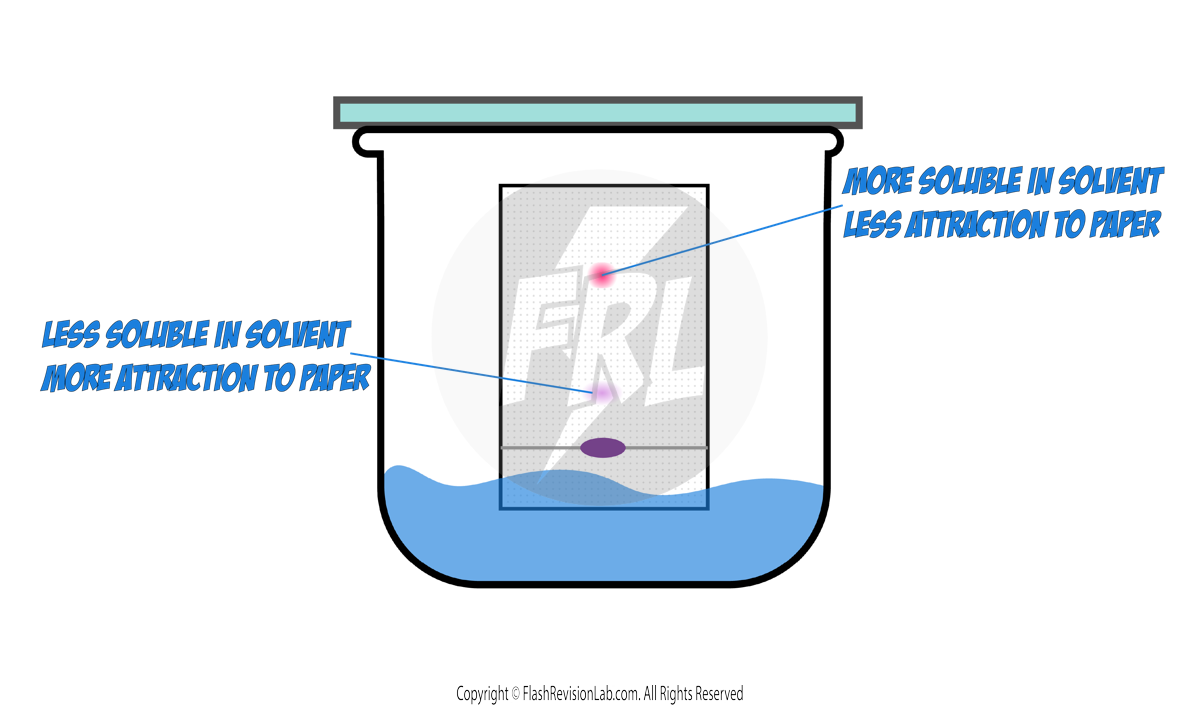
1. Solubility in the Mobile Phase
The MORE SOLUBLE a component is in the solvent, the MORE TIME it would spend in the MOBILE PHASE, which means it will travel FURTHER UP.
2. Attraction in the Stationary Phase
The MORE ATTRACTED a component is to the paper, the MORE TIME it would spend in the STATIONARY PHASE, which means it will NOT travel as far up.
Once the chromatography is done, the CHROMATOGRAM will show the different components in the mixture.
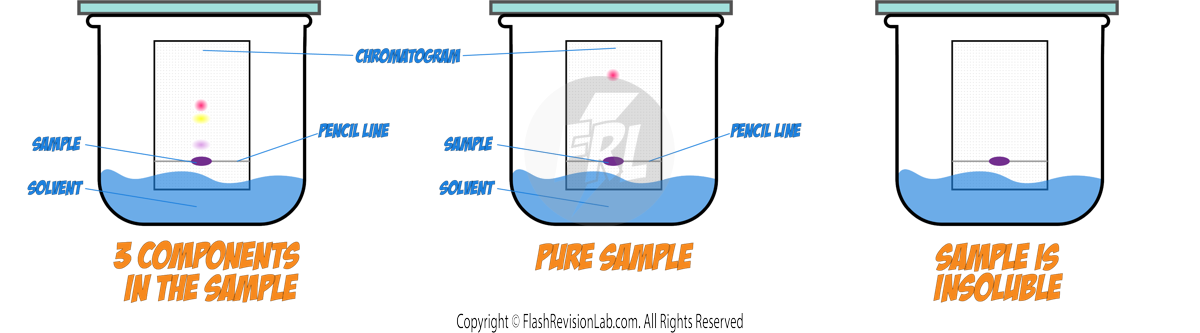
The number of SPOTS observed tells you the number of COMPONENTS in the mixture.
If there is only ONE SPOT on the chromatogram, the original sample is a PURE SUBSTANCE.
If the sample does NOT MOVE, it is INSOLUBLE in the solvent.
Rf Values
These values are used to IDENTIFY the COMPONENTS within a mixture.
They can be calculated using the following equation:

Heres an example:
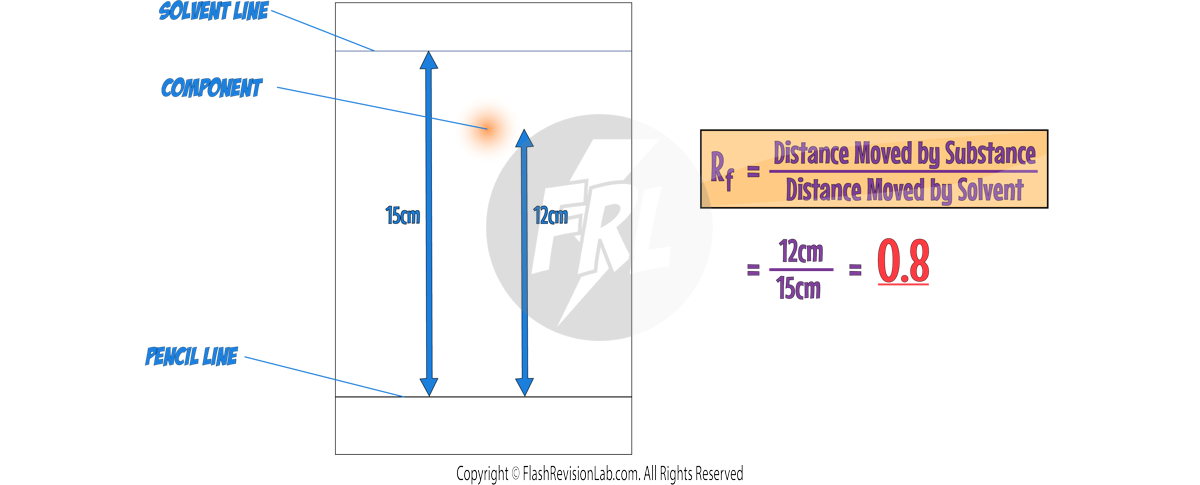
After you have the Rf value, you can compare it to data from REFERENCE MATERIALS to identify the component.
Chromatogram Analysis
Let's look at a chromatogram on an ink and 3 different dyes:
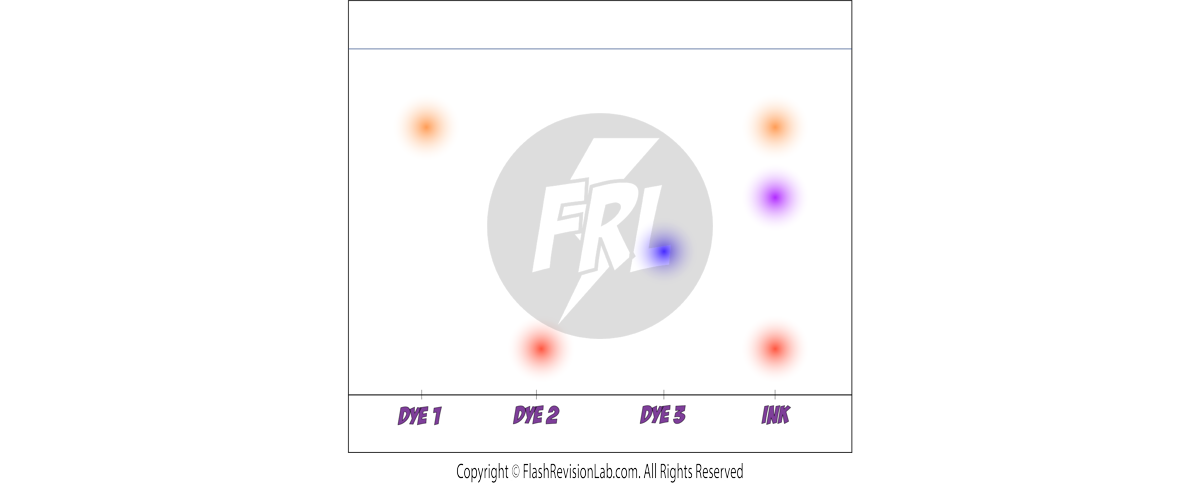
You can see that the ink has 3 DYES, two of which are known and one that is unknown.
You can tell by matching the heights of the components and assuming the ones at the SAME HEIGHTS have the same Rf values, so are the SAME SUBSTANCES.
Therefore, the ink contains DYE 1 and DYE 2 but does NOT contain DYE 3, as it is at a completely different height to the others.
Required Practical: Chromatography
You can carry out chromatography to separate and identify different components in a mixture such as INK or FOOD COLOURING.
Method
- Draw a PENCIL LINE near the bottom of the chromatography paper.
- Place dots of KNOWN DYE COLOURS and a dot of the INK being investigated.
- Place the bottom of the paper IN THE SOLVENT, making sure the pencil line is ABOVE the solvent.
- The solvent will move up the paper, taking the substances with it.
- Before the solvent reaches the top, remove the paper and let it dry to see the CHROMATOGRAM.
- Compare the positions of the dots on the KNOWN DYES to those of the INK.
Chromatogram Analysis
Let's look at a chromatogram on an ink and 3 different dyes:

You can see that the ink has 3 DYES, two of which are known and one that is unknown.
You can tell by matching the heights of the components and assuming the ones at the SAME HEIGHTS have the same Rf values, so are the SAME SUBSTANCES.
Therefore the ink contains DYE 1 and DYE 2 but does NOT contain DYE 3, as it is at a completely different height to the others.

Tests for Gases
You can test the presence of certain gases by carrying out the following tests:
1. Hydrogen
Add a LIT SPLINT to the gas.
If a SQUEAKY POP is observed, Hydrogen gas is present.
2. Oxygen:
Add a GLOWING SPLINT to the gas.
If the glowing splint RELIGHTS, Oxygen is present.
3. Carbon Dioxide
Bubble the gas through LIMEWATER.
If the limewater turns CLOUDY, the gas is Carbon Dioxide.
4. Chlorine:

Add DAMP BLUE LITMUS PAPER to the gas.
If the litmus paper BLEACHES WHITE, Chlorine is present.

The Early and Current Atmosphere
Composition of the Current Atmosphere:
The atmosphere we have today is the result of a long evolutionary process. Currently, it consists of:
- NITROGEN (N₂): Approximately 80%, the most common gas.
- OXYGEN (O₂): Roughly 20%, essential for life as we know it.
- TRACE GASES: These include CARBON DIOXIDE (CO₂), WATER VAPOUR (H₂O), and various NOBLE GASES like Argon, which are present in much smaller amounts.
The current atmosphere has had this composition for the last 200 MILLION YEARS, however it has not always been like this.
The Earth's Early Atmosphere:
Theories suggest that the Earth's original atmosphere (4.6 billion years ago) was very different from what it is today:

Volcanic Contributions:
- For the first billion years, the Earth was molten, and its atmosphere was formed from the gases given off by VOLCANOES. These gases likely included water vapor (H₂O), Carbon Dioxide (CO₂), Methane (CH₄), Ammonia (NH₃), and Nitrogen (N₂).
Ocean Formation and CO₂ Absorption:
- As the planet cooled, water vapour CONDENSED to form the OCEANS, which began ABSORBING CO₂. They also helped the formation of CARBONATE rocks, which created SEDIMENTS. This led to a REDUCTION in CO₂ levels in the atmosphere.
Early Atmospheric Conditions:
- Before life began, the Earth's atmosphere might have been similar to the atmospheres of Mars or Venus today — consisting of alot of Carbon Dioxide, with little to no Oxygen.
Nitrogen Accumulation:
- Nitrogen, released by volcanic activity, gradually built up in the atmosphere over time because it is relatively INERT (unreactive) and does not easily react to form new compounds.
Theories about what was in the Earth’s early atmosphere and how the atmosphere was formed have changed and developed over time. Evidence for the early atmosphere is limited because of the time scale of 4.6 BILLION YEARS.
The Development of the Oxygen Rich Atmosphere:
The presence of oxygen in the atmosphere is a result of life:
Around 2.7 billion years ago, ALGAE were the first organisms to PHOTOSYNTHESISE and began to use sunlight to convert Carbon Dioxide and water into Glucose and Oxygen.
- Word equation: CARBON DIOXIDE + WATER → GLUCOSE + OXYGEN
- Symbol equation: 6CO₂ + 6H₂O (light) → C₆H₁₂O₆ + 6O₂
This caused OXYGEN to be released into the atmosphere, and CARBON DIOXIDE to be REMOVED from the atmosphere.

Over the next billion years PLANTS began to evolve, which increased the levels of Oxygen and decreased the level of Carbon Dioxide even more.
The high levels of Oxygen allowed ANIMALS to evolve.
Animals fed on the plants which transferred Carbon to their tissues including bones and shells.
When these organisms died, their remains formed SEDIMENTARY ROCKS.
Some of the living organisms were buried in mud when they died.
Over millions of years, the heat and pressure turned the dead organisms into FOSSIL FUELS (crude oil, natural gas and coal).
The formation of sedimentary rock and fossil fuels 'LOCKED UP' the Carbon from Carbon Dioxide in the early atmosphere.
This is how the large amounts of Carbon Dioxide in the early atmosphere were reduced.

Greenhouse Gases and Climate Change
GREENHOUSE GASES like CARBON DIOXIDE, METHANE, and WATER VAPOUR are crucial for maintaining the Earth's temperature by trapping heat in the atmosphere. This is known as the GREENHOUSE EFFECT.
The Greenhouse Effect:
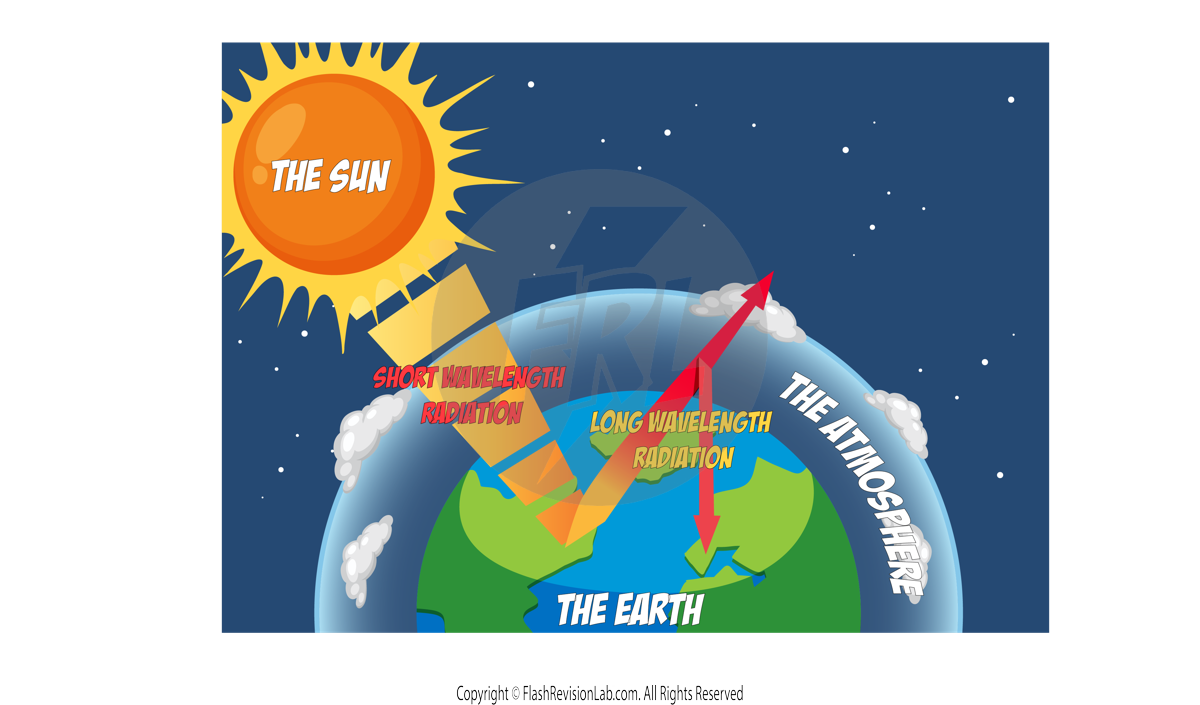
- RADIATION from the Sun reaches the Earth as SHORT-WAVELENGTH RADIATION, which is absorbed by the Earth's surface and then re-emitted as LONG-WAVELENGTH RADIATION.
- Some of this longer-wavelength radiation is absorbed and re-radiated by greenhouse gases in the ATMOSPHERE, warming the planet.
Human Impact on Greenhouse Gases:
Greenhouse gases in the atmosphere maintain temperatures on Earth high enough to support life, however human activities have increased their amounts. For example:
FOSSIL FUEL COMBUSTION

- Coal, crude oil and natural gas are all fossil fuels that humans use for transport and electricity generation.
- The COMBUSTION of these fuels released the ‘LOCKED UP’ Carbon into the atmosphere as CARBON DIOXIDE.
AGRICULTURE:

- Farm animals such as COWS release METHANE into the atmosphere due to their digestive processes.
DEFORESTATION:

- Cutting down trees has meant there are fewer trees that PHOTOSYNTHESISE. This means that LESS Carbon Dioxide is TAKEN IN from the atmosphere.
Global Climate Change
Too many greenhouse gases in the atmosphere INCREASES the greenhouse effect which can lead to GLOBAL WARMING.
This is when there is an INCREASE in the AVERAGE global temperature of the Earth.
This can lead to many problems such as:
- MELTING POLAR ICE CAPS.
- RISING SEA LEVELS leading to FLOODING.
- EXTREME WEATHER.
- LOSS of ANIMAL HABITAT.

Carbon Footprints
- CARBON FOOTPRINT measures the total amount of GREENHOUSE GASES emitted by an individual, service, event, or product over its entire lifecycle. This includes everything from production to disposal.
Strategies to Reduce Carbon Footprints
Reducing the Carbon footprint of something is important for tackling GLOBAL WARMING.
The main way of reducing your Carbon footprint is by reducing emissions of METHANE and CARBON DIOXIDE. Here are some effective strategies:
1. Individual Strategies:

- Using PUBLIC TRANSPORT instead of private cars.
- Reducing the consumption of MEAT.
- RECYCLING and REUSING materials.
2. Industrial Strategies:

- Transitioning to RENEWABLE ENERGY sources, such as solar or wind power, to replace fossil fuels.
- Implementing more ENERGY-EFFICIENT PROCESSES in industries and daily life.
- Introducing government policies that encourage reductions in greenhouse gas emissions, like Carbon taxing and setting emission CAPS.
- Developing and utilising CARBON CAPTURE technologies to trap CO2 before it is released into the atmosphere.
The Challenge of Making Reductions
- Despite knowing what needs to be done to reduce Carbon footprints, the actual implementation is complex due to ECONOMIC, POLITICAL, and SOCIAL factors.
- Individuals may not want to make changes to their LIFESTYLES. E.g. using public transport.
- Countries don’t want to sacrifice their ECONOMIC DEVELOPMENT if other countries don’t do the same, therefore it is difficult to achieve a GLOBAL AGREEMENT on the issue.
Pollution
FOSSIL FUELS like coal, oil and natural gas are major sources of HYDROGEN, CARBON and sometimes SULFUR. When burnt, they release:
- CARBON DIOXIDE (CO2).
- CARBON MONOXIDE (CO).
- WATER VAPOUR (H2O).
- OXIDES OF NITROGEN (NO or NO2).
- SULFUR DIOXIDE (SO2).
- SOOT- CARBON PARTICULATES (C).

CARBON DIOXIDE AND WATER VAPOUR:
These are produced by the COMPLETE COMBUSTION of hydrocarbons.
They are GREENHOUSE GASES that can lead to GLOBAL WARMING.
CARBON MONOXIDE:
This is a TOXIC GAS produced by INCOMPLETE COMBUSTION of hydrocarbons.
If breathed in, can bind to HAEMOGLOBIN in RED BLOOD CELLS which can stop the BLOOD from CARRYING OXYGEN.
This can lead to FAINTING, COMA and even DEATH.
It is an ODOURLESS and COLOURLESS gas making it difficult to detect.
CARBON PARTICULATES:
Also known as SOOT, SOLID pieces of Carbon can be formed during INCOMPLETE COMBUSTION.
When inhaled, it can lead to RESPIRATORY PROBLEMS.
When released into the environment, it can lead to GLOBAL DIMMING where LESS LIGHT reaches the Earth.

SULFUR DIOXIDE:
This is produced during the combustion of COAL containing SULFUR IMPURITIES.
The Sulfur reacts with Oxygen to form SULFUR DIOXIDE.
When this mixes with water in the clouds, it forms SULFURIC ACID which falls as ACID RAIN.
ACID RAIN can kill plants, and damage BUILDINGS and STATUES.
It can also cause RESPIRATORY PROBLEMS when inhaled.
OXIDES OF NITROGEN:

This can include gases like NO and NO2, which are formed in CAR ENGINES. The HIGH TEMPERATURES created by the burning of the fossil fuels allow NITROGEN and OXYGEN in the air to REACT to form OXIDES OF NITROGEN.
These can mix with water in clouds to form NITRIC ACID, which can fall as ACID RAIN.
These can also cause RESPIRATORY PROBLEMS when inhaled.
Earth's Resources and Sustainability
Utilising Earth's Resources
Humans use the EARTH'S RESOURCES for many essential aspects of life. These are NATURAL RESOURCES that have been made WITHOUT any human input, and we use these for WARMTH, SHELTER, FOOD and TRANSPORT. Here are some examples:

- WARMTH:
- Natural Gas: Widely used for HEATING homes and WATER.
- Firewood: Traditional source of HEAT, particularly in rural areas.
- SHELTER:
- Stone: Used in the construction of durable and weather-resistant STRUCTURES.
- Clay: Utilised for making BRICKS and tiles for building homes.
- Sand: Used to make CONCRETE, which is important in modern construction.
- FOOD:
- Soil: The foundation for growing CROPS and supporting agriculture.
- Fish: A natural source of nutrition, sourced from oceans, rivers, and lakes.
- TRANSPORT:
- Crude Oil: Converted into fuels like petrol and diesel, crucial for powering vehicles.
- Iron Ore: Used to produce steel, an integral material for vehicle construction.
The Earth’s Natural resources are often IMPROVED by MAN-MADE processes such as AGRICULTURE and SYNTHETIC PROCESSES. Here are some examples:
AGRICULTURALLY IMPROVED PRODUCTS

FOOD:
- CROP production has been INCREASED by the introduction of FERTILISERS.
- WILD-CAUGHT FISH are supplemented by FARM-RAISED FISH.
TIMBER:
- Naturally grown FORESTS are supplemented by managed timber PLANTATIONS that provide a continuous supply of wood for construction and paper production.
CLOTHING:
- Natural WOOL or SILK is supplemented by agriculturally produced cotton and hemp fabrics.
FUELS:
- Traditional WOOD LOGS for burning are supplemented by agricultural products such as corn ethanol or BIOFUELS made from various plant materials.
- Biodiesel produced from agricultural crops like rapeseed and soybean oil can supplement or replace petroleum diesel.
SYNTHETICALLY IMPROVED PRODUCTS
- Cotton: A natural fibre often supplemented by synthetic fibres like POLYESTER in clothing.
- Rubber: Natural rubber can be supplemented or replaced by synthetic rubber, commonly used in tyres and industrial products.
- Leather: Natural leather can be replaced with synthetic leather in furniture, clothing, and accessories.
- Wood: Natural wood in construction can be supplemented with engineered wood products like plywood.
Finite and Renewable Resources
There are two main types of resources:
1. FINITE RESOURCES:
- These are resources that will EVENTUALLY RUN OUT. They come from the EARTH, OCEANS, and ATMOSPHERE and include fossil fuels, metal ores and minerals. Once used, they cannot be replaced instantly.
2. RENEWABLE RESOURCES:
- Unlike finite resources, renewable resources can replenish naturally over time. Examples include solar energy, wind energy, and wood from managed forests.

The Role of Chemistry
CHEMISTRY is vital in the process of CONVERTING NATURAL RESOURCES into useful products. It plays a central role in:
- IMPROVING AGRICULTURAL PROCESSES: Chemistry helps in the development of fertilisers and pesticides, which increase food production.
- CREATING SUSTAINABLE MATERIALS: Through Chemistry, we can develop materials that meet our needs while ensuring that future generations can also meet theirs.
Sustainable Development
SUSTAINABLE DEVELOPMENT is a developmental approach that considers the long-term impact on the environment. Its goal is to create products that fulfil our current needs without depleting resources or harming the environment for future generations.
Potable Water
- POTABLE WATER is another term for water that's safe to drink.
- This means it contains very LOW levels of dissolved SALTS and microbes, and is free from harmful chemical substances.
- It's not the same as PURE WATER, which ONLY contains H2O molecules, whereas potable water CAN include various dissolved substances.
Producing Potable Water from Fresh Water
There are THREE steps to obtain POTABLE WATER from FRESH WATER:
1. CHOOSING WATER SOURCES:
- The selection of water sources is crucial. In places like the UK, water providers usually collect water with low levels of dissolved substances from FRESH WATER sources such as SURFACE WATER or GROUNDWATER.
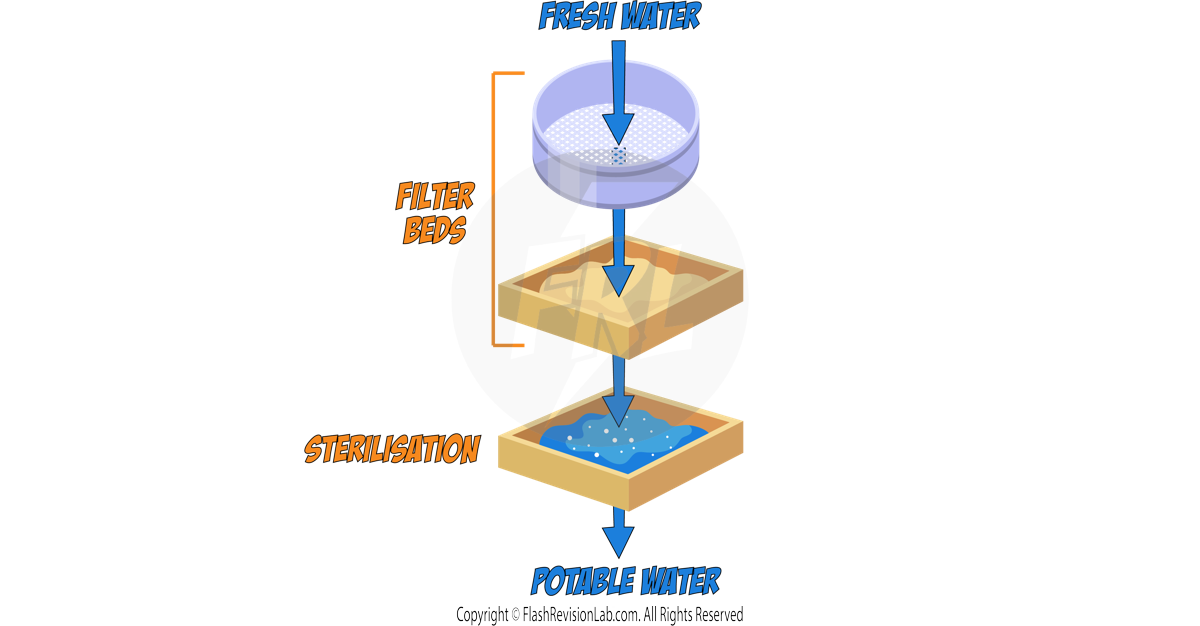
2. PASSING WATER THROUGH FILTER BEDS:
- This involves passing the water through a WIRE MESH or SAND BEDS to help remove solid particles.
3. STERILISING:
- To make water safe from harmful MICROBES, it's treated with sterilising agents like CHLORINE, OZONE, or exposed to ULTRAVIOLET LIGHT.
Dealing with Limited Fresh Water Supplies
The methods used to produce potable water depend on available supplies of water and local conditions.
For example, the UK primarily uses GROUNDWATER due to its DRIER Southeast region which leads to LESS SURFACE WATER.
Producing Potable Water from Salty Water
In places where there is LESS FRESH WATER available, alternative methods like DESALINATION of salty water or sea water are used. This involves removing SALTS from water to make them POTABLE.
DESALINATION can be done in TWO different ways:
DISTILLATION:
- This involves BOILING water to create steam and then CONDENSING it back into liquid, leaving the salts behind.
REVERSE OSMOSIS:
- A method involving a MEMBRANE which allows water particles to pass through it but NOT salt ions, meaning they can be separated.
Both desalination processes typically require a lot of ENERGY, so are EXPENSIVE processes.

Required Practical: Analysis and Purification of Water
There are TWO experiments in this practical:
1. Finding the Amount of Dissolved Solid in a Water Sample
If a water sample has dissolved solid in it, you can EVAPORATE the water out, leaving behind just the solids.
Method:
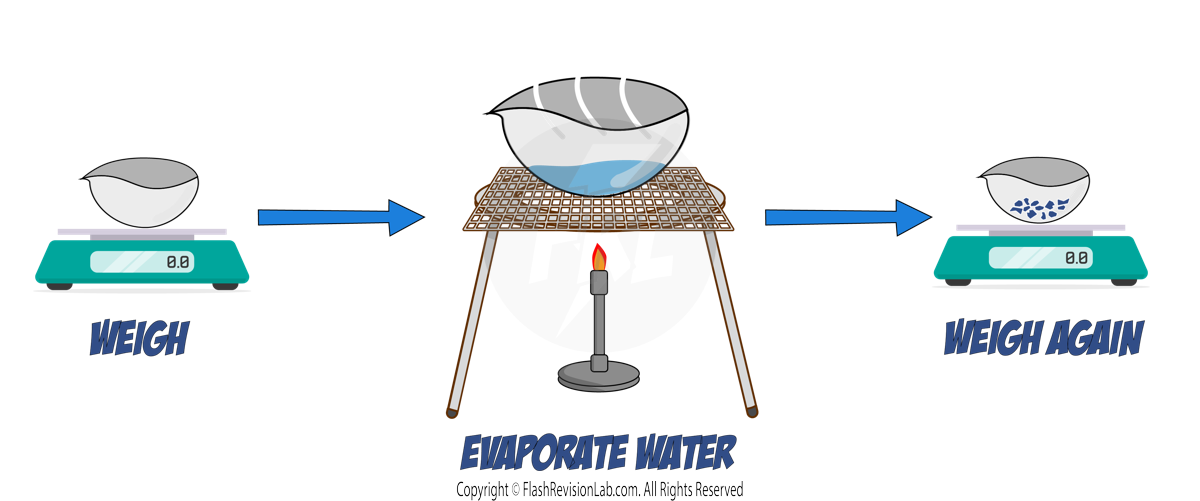
1. Record the MASS of a dry evaporating basin using a MASS BALANCE.
2. Pour a known volume of the water sample into the EVAPORATING BASIN.
3. Heat the evaporating basin until its mass stays constant (all the water has evaporated).
4. Record the mass of the evaporating basin and contents.

Now you can SUBTRACT the mass at the end from the mass at the start to get the MASS of the DISSOLVED SOLIDS.
2. Purifying Water
To get a PURE sample of water you can use the DISTILLATION method.
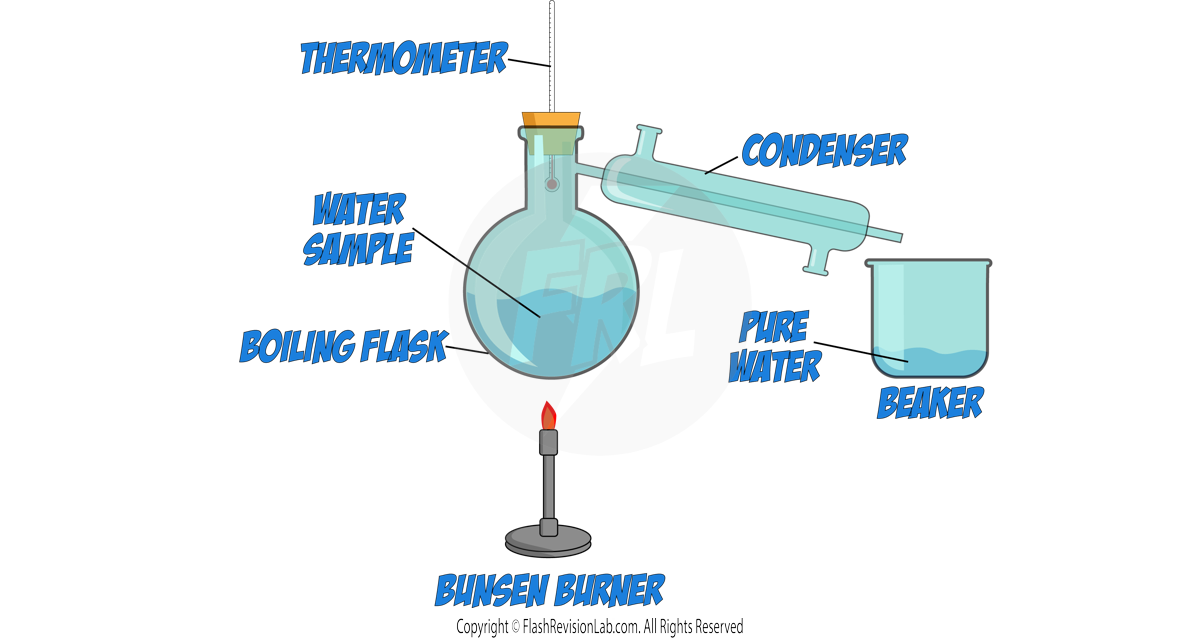
Method:
1. Add the water sample to the boiling flask.
2. HEAT the water using the Bunsen burner until boiling occurs.
3. The water vapour will pass through the condenser and CONDENSE.
4. The DISTILLED water will collect in the beaker.
Results:
You can ANALYSE the water you have collected by:
- Checking the pH by using a pH METER. It should have a pH close to 7.
- Checking the PURITY by checking the BOILING POINT. It should be 100°C.

Waste Water Treatment
- Waste water comes from various HOUSEHOLD ACTIVITIES, such as bathing, washing dishes, and flushing toilets. This water typically goes into SEWERS.
- AGRICULTURAL RUNOFF and industrial processes also contribute significantly to waste water.
- Waste water must be treated before being returned to the environment.
- Sewage and agricultural waste water require removal of organic matter and harmful MICROBES.
- Industrial waste water may require removal of organic matter and harmful CHEMICALS.
Sewage Treatment Process
There are several stages to the treatment of waste water:
- SCREENING & GRIT REMOVAL: Large objects, such as twigs and plastic, are removed.
- SEDIMENTATION: Waste water is stored in a large tank allowing heavy solids to settle, known as SLUDGE, while lighter substances called EFFLUENT float to the top.
- AEROBIC BIOLOGICAL TREATMENT: Effluent is treated with aerobic BACTERIA that consume organic matter.
- ANAEROBIC DIGESTION: Sludge from the sedimentation tank is broken down by bacteria in a no-Oxygen environment, producing METHANE and FERTILISERS.
- Additional treatment is necessary for water contaminated with hazardous chemicals, which may include UV RADIATION or MEMBRANE FILTRATION.
Sewage treatment requires MORE PROCESSES than treating fresh water but USES LESS ENERGY than the DESALINATION of salt water, so could be used as an alternative in areas where there's not much fresh water.

Alternative Methods of Metal Extraction
Metal ores are FINITE RESOURCES, meaning they will eventually RUN OUT.
As METAL ORES become harder to find and extract, alternative methods of extraction are becoming more important.
Traditional mining involves digging, moving and disposing of large amounts of rock which can DAMAGE the ENVIRONMENT.
There are TWO methods that can extract LOW GRADE ORES (rocks with a small amount of metal) while minimising damage to the environment:
1. Phytomining
PHYTOMINING uses plants to absorb METAL COMPOUNDS from the soil. The steps involved are:
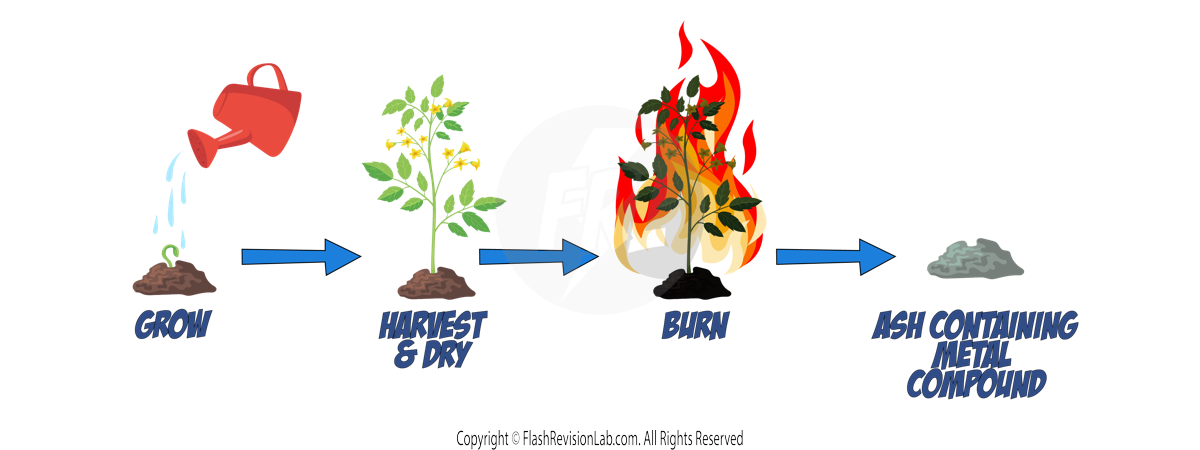
- GROWING PLANTS: Plants that can absorb metal compounds are grown on soil that contains these compounds.
- HARVESTING: Once the plants have absorbed the metals, they are harvested and dried.
- PROCESSING: The harvested plants are then BURNED to produce ASH that contains the metal compounds.
2. Bioleaching
BIOLEACHING involves using bacteria to extract metals. The process is as follows:
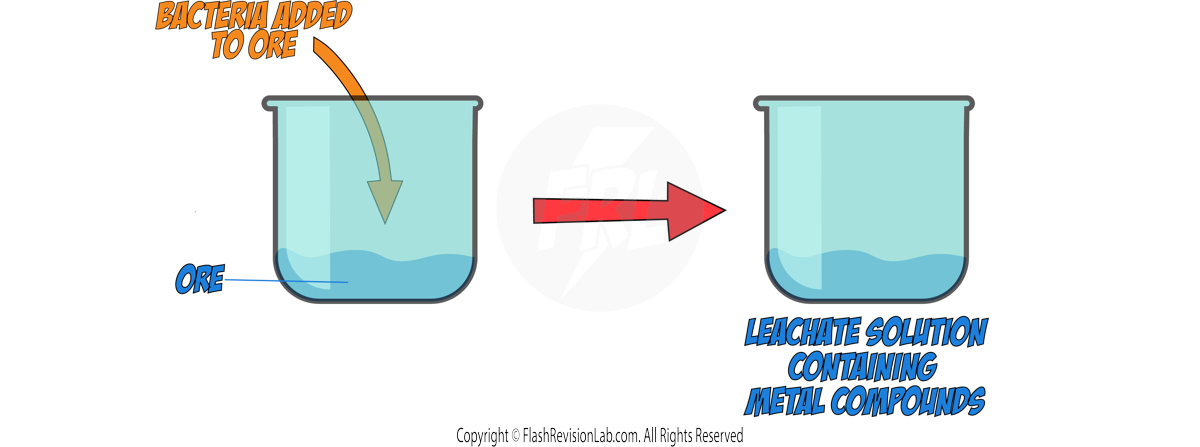
- BACTERIA APPLICATION: Bacteria are introduced to the ore where they produce LEACHATE solutions that contain metal compounds.
- COLLECTING LEACHATE: The leachate is collected as it contains the desired metals.
Extracting the Metals
Once you have the metal compounds from phytomining or bioleaching, you can DISSOLVE THEM IN ACID and they can be processed to extract the pure metal. This can be done by:
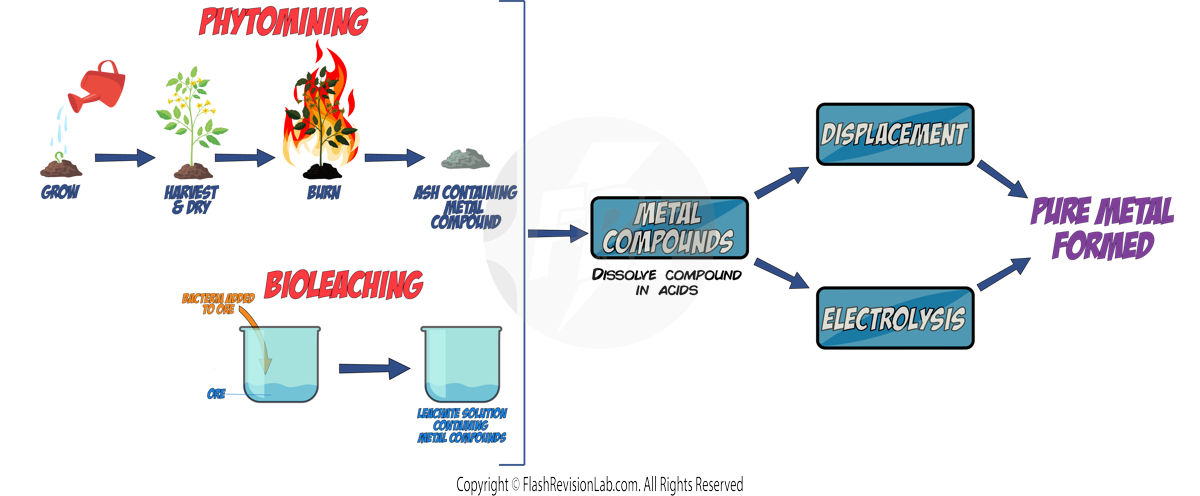
- DISPLACEMENT: Using a more reactive metal, like scrap iron, to displace the metal from its compound.
- ELECTROLYSIS: An electrical process that separates the metal from its compound.
Life Cycle Assessments
A LIFE CYCLE ASSESSMENT (LCA) is a method used to evaluate the environmental impact of a product through all stages of its life. This includes:
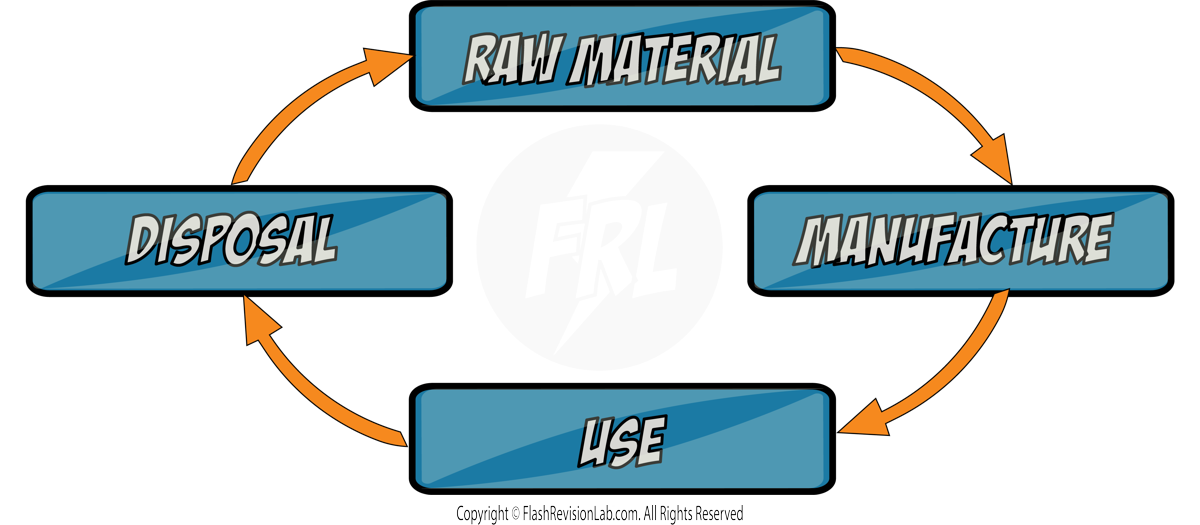
- EXTRACTING AND PROCESSING RAW MATERIALS: The initial stage where materials are collected from the environment.
- MANUFACTURING AND PACKAGING: The process of turning raw materials into finished products and preparing them for sale.
- USE AND OPERATION DURING ITS LIFETIME: How the product is used by consumers and how efficiently it operates.
- DISPOSAL AT THE END OF ITS USEFUL LIFE: What happens to the product after it can no longer be used, including the impacts of transporting the product to disposal facilities and the disposal process itself.
Example of LCAs:
You can compare the environmental impacts of SHOPPING BAGS made from plastic and paper.
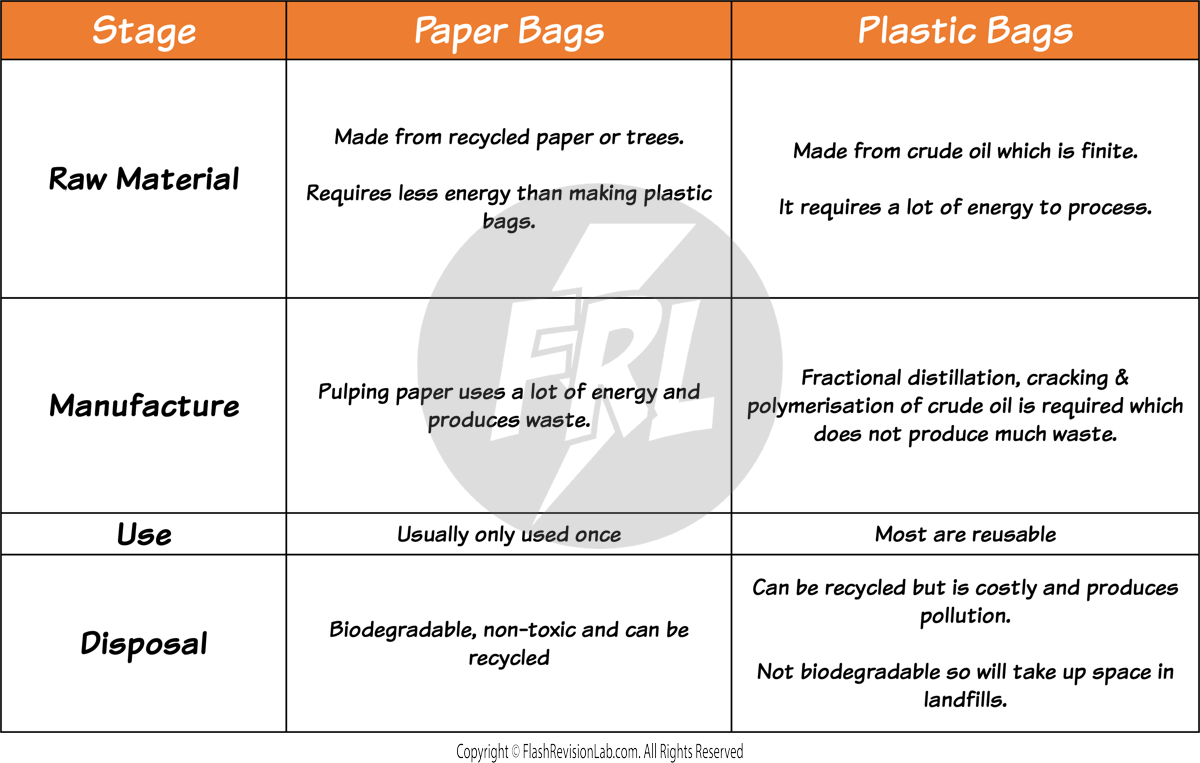
From this LCA, you can conclude that even though plastic bags are NOT biodegradable, they take LESS energy to make, and have a LONG LIFESPAN as they can be reused. This could mean that they are LESS damaging to the environment.
Quantifying Environmental Impact
In an LCA, various factors can be quantified with NUMBERS to understand a product's impact:
- WATER USAGE: The amount of water required in the product's lifecycle.
- RESOURCE CONSUMPTION: The raw materials needed for the product.
- ENERGY SOURCES: The types and amounts of energy used at each stage.
- WASTE PRODUCTION: The waste generated, including its management.
While these can often be measured in numbers, some effects such as POLLUTANT EFFECTS are more complex so it involves SUBJECTIVE measurements based on a person's JUDGEMENT. This can lead to BIAS.
Abbreviated LCAs
Sometimes, ABBREVIATED or SELECTIVE LCAs are conducted. These are simplified versions that may not include all stages of the lifecycle or may focus on specific aspects of environmental impact. However, they must be used carefully to avoid misleading conclusions, especially in advertising.
Reuse and Recycling
Learning how to manage our resources effectively is crucial for environmental sustainability. The principles of REDUCTION, REUSE, and RECYCLING—often referred to as the "3 R's"—are key strategies in conserving resources and minimising waste.

REDUCTION:
The most effective way to minimise waste is to not create it in the first place. Reducing our consumption leads to fewer materials needing to be recycled or disposed of, thereby saving energy and reducing pollution.
REUSE:
Before throwing things away, consider if they can serve another purpose. Products like GLASS BOTTLES are perfect for reuse, helping to cut down on the need for new materials and the energy required to produce them.
RECYCLING:
Transforming used materials into new products is essential for reducing the need for raw resources and the energy used in production processes.
Recycling Specific Materials
Different materials require different approaches when it comes to recycling. Understanding the specific processes for materials like metals, glass, and plastics is important for efficient recycling practices.

METALS:
The process of recycling metals saves precious raw materials and reduces the vast energy requirements of metal production.
GLASS:
Simple yet effective, the recycling of glass conserves sand and reduces the manufacturing energy cost significantly.
BUILDING MATERIALS:
Reclaimed building materials can significantly reduce the environmental footprint of new construction projects.
CLAY CERAMICS AND PLASTICS:
Despite being challenging, recycling these materials can lead to a substantial decrease in environmental degradation and resource depletion.